Final ID: WP97
Derivation and Validation of the Get with the Guidelines®-Stroke Endovascular Thrombectomy Risk Scores
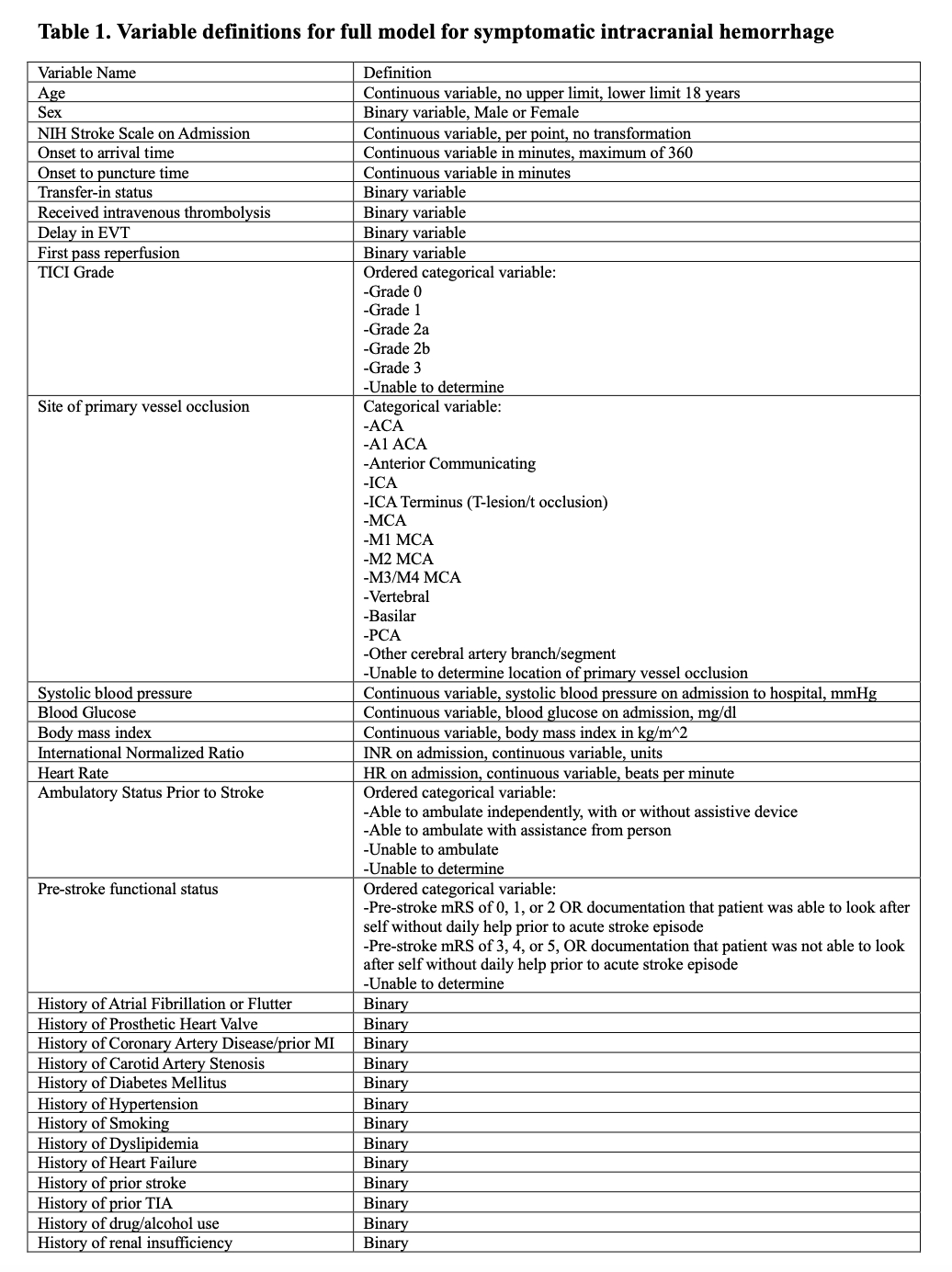
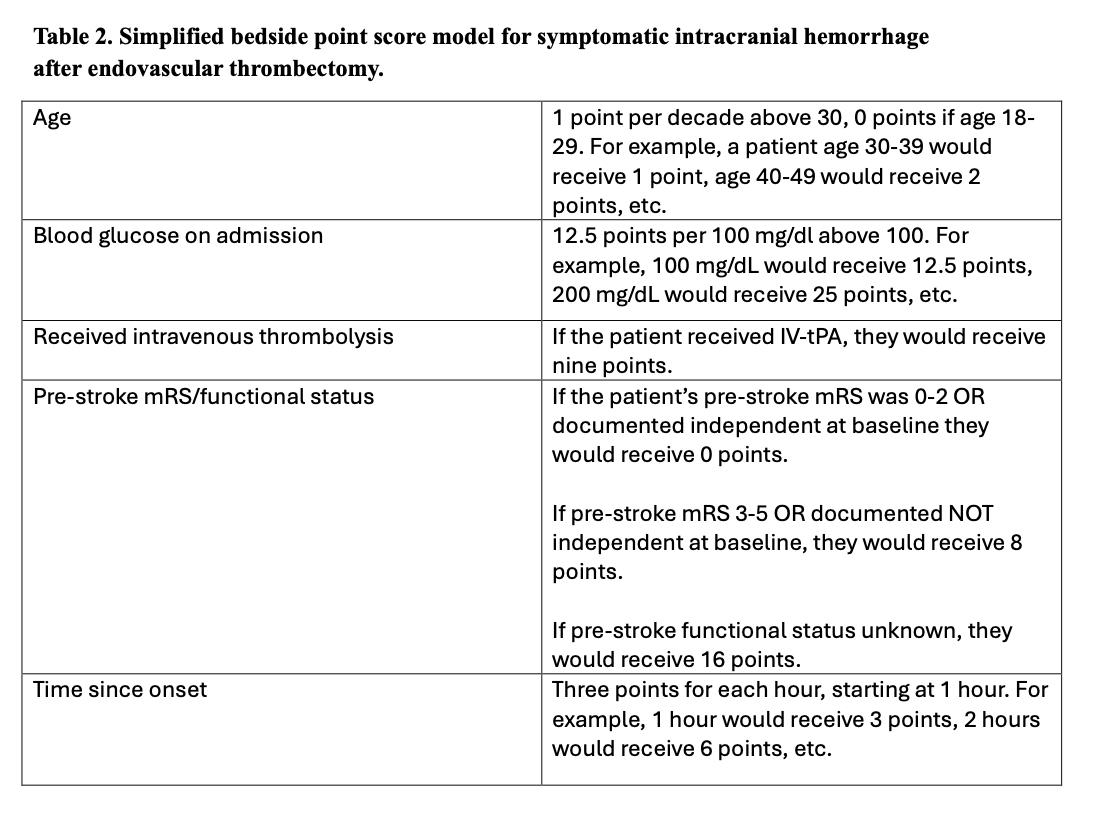
Methods: Patients presenting to GWTG-Stroke participating hospitals between July 2021 and June 2023 with last known well within six hours prior to presentation, who received endovascular thrombectomy were included. The primary outcome was sICH; secondary outcomes included in-hospital mortality, mRS at discharge, and length of stay. The study population was divided into a derivation and validation cohort with 70:30 partition. According to a pre-specified statistical analysis plan, a full model of 31 candidate variables and subsequently a highly parsimonious model including only variables measured before EVT deployment was fit for each endpoint, with variable retention guided by multiple factor analysis (MFA). Models were then externally validated in the HERMES clinical trial population.
Results: 31,668 patients (median age 71 [Q1: 61, Q3: 81]) were included, of whom 1,799 (5.7%) developed sICH. In the validation cohort, the area under the receiver operating characteristics curve (AUC) for the full model was 0.649 (Table 1), and the AUC for the simplified points score was 0.589 (Table 2). At the conference, we will present results of external validation and secondary endpoints, details of model calibration, and direct comparisons to existing risk scores.
Conclusions: A risk score for sICH after thrombectomy for acute stroke devised using routinely collected data known prior to intervention had good performance compared to existing approaches.
More abstracts on this topic:
Stoehr Kaitlyn, Matouk Charles, Hebert Ryan, Gilmore Emily, Kim Jennifer, Petersen Nils, Jayasundara Sithmi, Vargas David, Thinzar Pwint, Rapuano Amedeo, Maarek Rafael, Beekman Rachel, Magid-bernstein Jessica, Okeefe Lena
A Phase 2 Study Evaluating the Effects of Mivelsiran, an Investigational RNA Interference Therapeutic, on Hemorrhagic and Nonhemorrhagic Manifestations of Cerebral Amyloid AngiopathyGreenberg Steven, Parikh Neal, Lee Jin-moo, Van Etten Ellis, Van Osch Matthias, Klijn Catharina, Sostelly Alexandre, Goteti Sasikiran, Sepehrband Farshid, Avbersek Andreja, Deering Robert
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.