Final ID: 144
Mobile Stroke Unit Management and Outcomes in Undifferentiated Patients with Stroke-Like Symptoms in the Prehospital Setting: A Nationwide Cohort Study
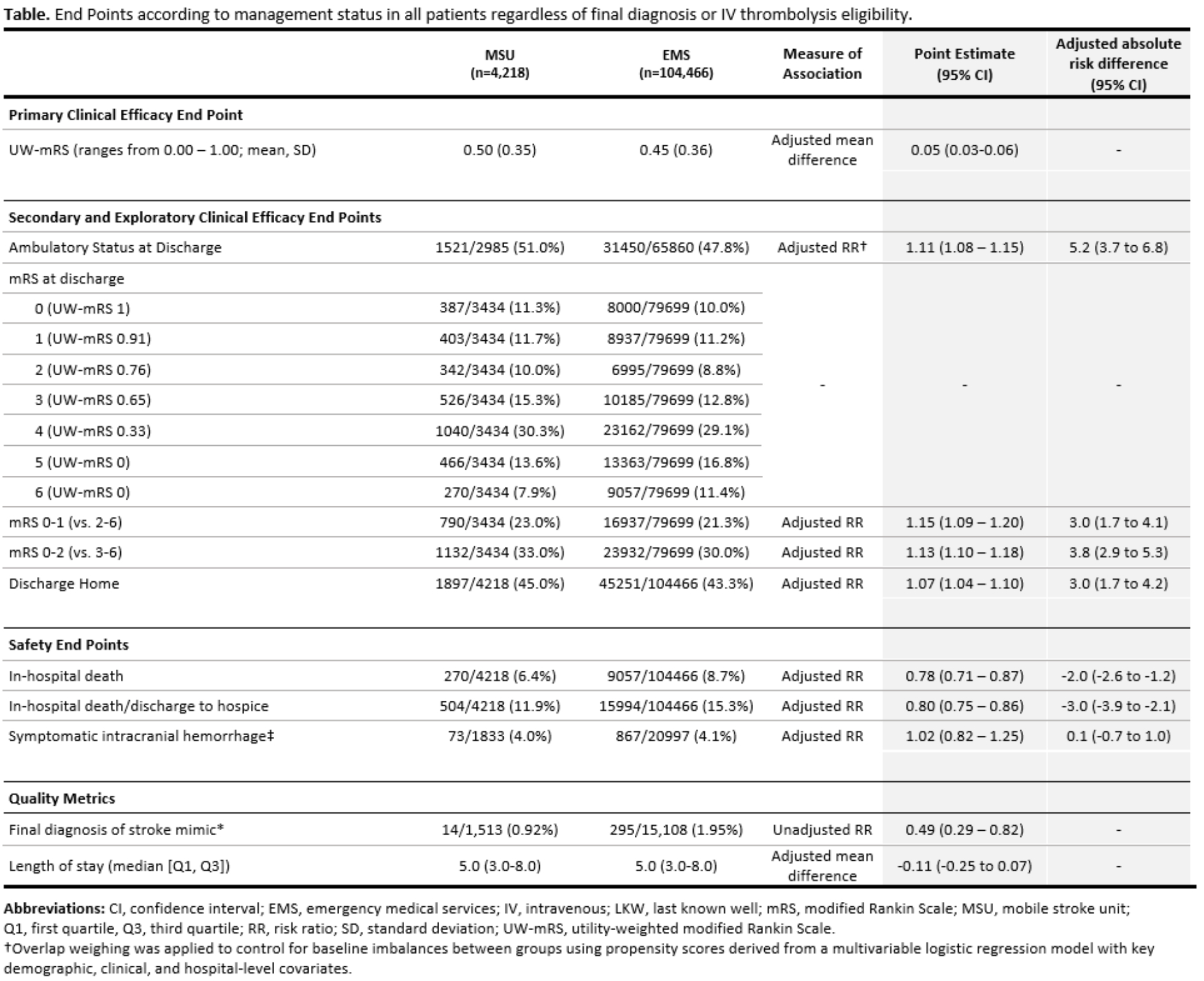
Methods: We performed a retrospective, observational, cohort study from the American Heart Association’s Get With The Guidelines®-Stroke (GWTG-Stroke) Program between August 1st 2018 and January 31st 2023. At participating hospitals receiving both MSU-managed and standard EMS-managed patients, all patients with stroke-like symptoms in the prehospital setting were analyzed. The primary efficacy end point was the level of global disability assessed with the utility-weighted modified Rankin Scale (UW-mRS; rage 0.00 – 1.00). The secondary efficacy end point was ambulatory status. The co-primary safety end points were symptomatic intracranial hemorrhage (sICH) and in-hospital mortality.
Results: Of 108,684 patients (median age 73 [Q1,Q3: 62,83]; 51.2% female), 4,218 (3.9%) received prehospital management in an MSU. Prehospital management in an MSU was associated with a better score on the UW-mRS at discharge (adjusted mean difference 0.05 [95% CI: 0.03 – 0.06]) and a higher likelihood of independent ambulation at discharge (51.0% [1,521/2,985] vs. 47.8% [31,450/79,699]; aRR 1.11 [95% CI: 1.08 – 1.15]; adjusted risk difference 5.2% [95% CI: 3.7 to 6.8]). There was no statistically significant difference in sICH (4.0% vs. 4.1%; aRR 1.02 [95% CI: 0.82 – 1.25]). There was a lower rate of in-hospital mortality (6.4% vs. 8.7%; aRR: 0.78 [95% CI: 0.71 to 0.87]) in MSU-treated patients.
Conclusions: Among patients with stroke-like symptoms in the prehospital setting, prehospital management in an MSU compared with EMS management was associated with a significantly lower level of global disability. These findings support policy efforts to expand access to MSU care in the United States.
More abstracts on this topic:
Lee Ho-joon, Schwamm Lee, Turner Ashby, De Havenon Adam, Kamel Hooman, Brandt Cynthia, Zhao Hongyu, Krumholz Harlan, Sharma Richa
Association of Biomarkers with risk of Hematoma Expansion and Arterial Thromboembolic Events in Acute Factor Xa Inhibitor-Associated Intracerebral Hemorrhage: The ANNEXa-I Biomarker SubstudyShoamanesh Ashkan, Verhamme Peter, Eikelboom John, Sharma Mukul, Xu Lizhen, Bamberg Krister, Beyer-westendorf Jan, Falkenberg Cecilia, Ladenvall Per, Narayan Rohit, Penland Robert
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.