Final ID:
Main Results of the Cooperative Program for ImpLementation of Optimal Therapy in Heart Failure (COPILOT-HF) Program – A Pragmatic Randomized Study
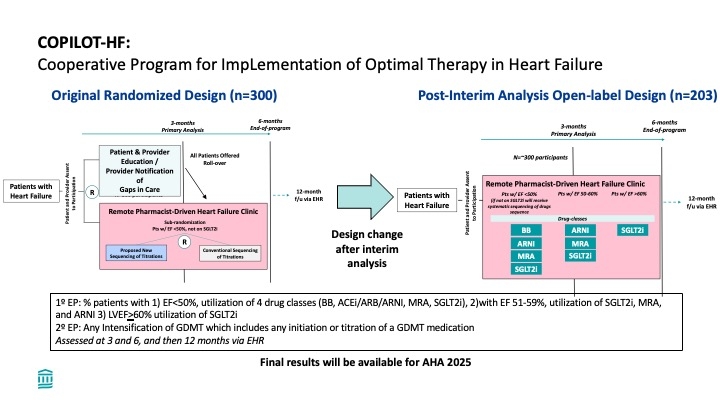
Methods: The Cooperative Program for Implementation of Optimal Therapy in Heart Failure (COPILOT-HF) (NCT05734690) is a pragmatic, randomized, open-label clinical trial comparing two strategies for remote GDMT optimization across the spectrum of HF, regardless of LVEF. Participants (pts) were randomized 1:1 to either a 3-month education-first strategy followed by medication management or simultaneous initiation of both interventions (Figure). The primary efficacy endpoint is the percentage of pts receiving full guideline-directed HF treatment at 3 months. Safety outcomes include kidney complications, hypotension, hospitalizations, and deaths. A prespecified interim analysis was performed after the first 100 pts completed 4 months in the program.
Results: 503 HF pts were enrolled. Mean age was 72yrs, 53% were male and 89% white. Hypertension (87%), obesity (mean BMI 32 kg/m2) and T2D (32%) were common comorbidities, and 29% were hospitalized for HF within 1 year of enrollment. Mean baseline labs were NTproBNP 1302 pg/ml, potassium 4.3 mEq/L, and eGFR 68.6 mL/min/1.73 m2. A total of 174 pts (35%) had an LVEF <50% (including 16% with EF<40%), 26% had EF 50–59%, and 40% had EF≥60%. Baseline medications were: 89% B-blocker, 33% MRA, 32% SGLT2i, and 30% ARNI for EF<50%, and 77% B-blocker, 18% MRA, 2% SGLT2i, and 4% ARNI for EF>=50%. At the interim analysis, 4 pts (8%) in the Education-first arm achieved the 1° EP compared to 27 (54%) in the simultaneous arm (p<0.001). Based on these results, all subsequent pts were enrolled into a single-arm program of simultaneous medication management and education. The last pts was enrolled in February 2025. Navigators completed 16,536 tasks (mean 33.1 per pts ) facilitating patient progress through the program with 1,966 medications (mean 3.9 per pts ) initiated, and 2,967 labs (mean 5.9 per pts ) reviewed. While enrolled, 70 pts were hospitalized, including 10 hospitalized for heart failure.
Conclusions: A strategy of remote medication implementation combined with education appeared to result in a higher proportion of pts with HF receiving optimal medical therapy compared with education first. Final data will be available for presentation at AHA 2025.
- Blood, Alexander ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Mcpartlin, Marian ( Mass General Brigham , Somerville , Massachusetts , United States )
- Wagholikar, Kavishwar ( Massachusetts General Hospital , Westborough , Massachusetts , United States )
- Caberwal, Harjeet ( BIPI , Howell Township , New Jersey , United States )
- Cannon, Christopher ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Desai, Akshay ( BRIGHAM WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Scirica, Benjamin ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Unlu, Ozan ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Hassan, Shahzad ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Nichols, Hunter ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Ostrominski, John ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Subramaniam, Samantha ( Mass General Brigham , Somerville , Massachusetts , United States )
- Zelle, David ( Mass General Brigham , Somerville , Massachusetts , United States )
- Figueroa, Christian ( Mass General Brigham , Somerville , Massachusetts , United States )
- Varugheese, Matthew ( Mass General Brigham , Somerville , Massachusetts , United States )
Meeting Info:
Session Info:
Biological and Pragmatic Interventions in Heart Failure: From Present to Future
Sunday, 11/09/2025 , 08:00AM - 09:15AM
Featured Science
More abstracts on this topic:
Chai Jocelyn, Sathananthan Janarthanan, Fine Nowell, Davis Margot, Starovoytov Andrew, Campbell Christine, Hawkins Nathaniel, Virani Sean, Luong Michael, Straatman Lynn, Kiess Marla, Worsley Daniel
A Growing Burden of Electronic Medical Record Messages in ACHD CareDailey Schwartz Andrew, Alegria Jorge
More abstracts from these authors:
Unlu Ozan, Oates Michael, Cannon Christopher, Scirica Benjamin, Wagholikar Kavishwar, Aronson Samuel, Blood Alexander, Varugheese Matthew, Shin Jiyeon, Subramaniam Samantha, Stein David, St.laurent John, Mailly Charlotte, Mcpartlin Marian, Wang Fei
Implementation and Evaluation of an Electronic Health Record Rule-Based System to Identify Heart Failure Patients Eligible for Guideline-Directed Medical TherapyHassan Shahzad, Collins Emma, Figueroa Christian, Ruggiero Ryan, Fridley Echo, Varugheese Matthew, Gabovitch Dan, Cannon Christopher, Desai Akshay, Blood Alexander, Scirica Benjamin, Subramaniam Samantha, Wagholikar Kavishwar, Kumar Sanjay, Unlu Ozan, Zelle David, Ostrominski John, Nichols Hunter, Mcpartlin Marian, Twinning Megan