Final ID: Mo3088
Implementation and Evaluation of an Electronic Health Record Rule-Based System to Identify Heart Failure Patients Eligible for Guideline-Directed Medical Therapy
Despite well-established benefits on morbidity and mortality, implementation of guideline-directed medical therapy (GDMT) in heart failure (HF) population remains suboptimal. Quality improvement interventions to improve GDMT uptake often necessitate labor-intensive manual chart reviews to identify eligible patients. To automate this process, we implemented and evaluated a Rule-Based System (RBS) designed to identify HF patients eligible for remote medication optimization program.
Methods
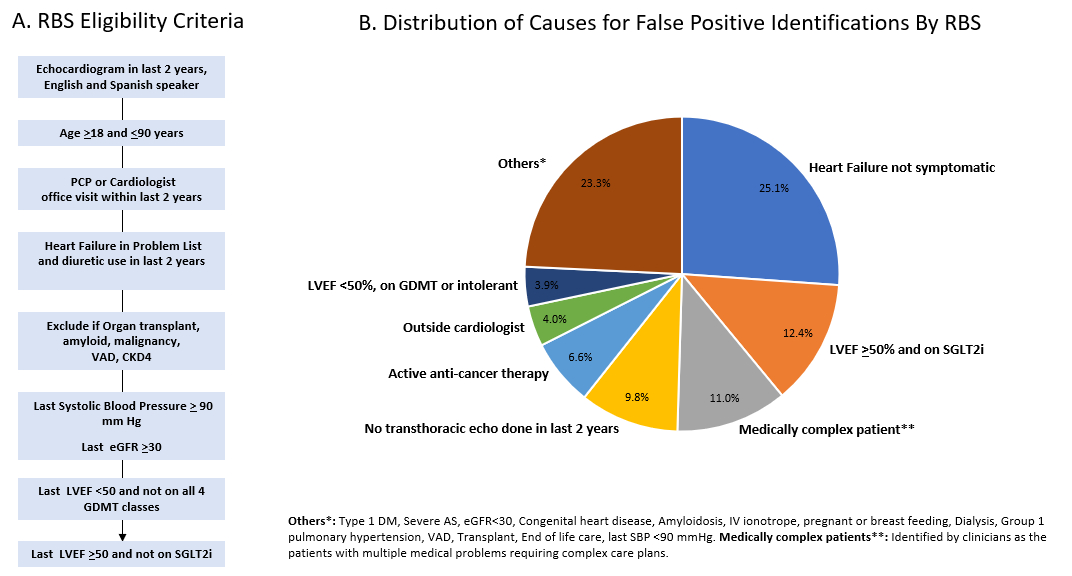
To identify symptomatic HF patients for enrollment in our Cooperative Program for ImpLementation of Optimal Therapy in Heart Failure (COPILOT-HF), an RBS was hierarchically developed to first identify HF patients meeting program criteria (Figure 1A), then exclude those with contraindications or at increased risk of adverse drug reactions. The RBS processed structured data from electronic health records (EHR) from Mass General Brigham’s enterprise data warehouse to compile a list of potentially eligible patients as per program criteria. This list was then reviewed by program staff, who manually verified patient eligibility.
Results
A total of 5,460 patients were identified by the RBS as potentially eligible, of whom 1,754 (32.1%) were confirmed as eligible on manual chart review with positive predictive value of 47.3%. Among the false positives, 25% did not meet the program criteria for symptomatic HF, 12.4% with LVEF >50% were already on SGLT2i, 11.0% were too medically complex for our program, 9.8% did not have a recorded echocardiogram within 2 years, 6.6% were receiving anticancer therapies, 4.0% had outside cardiologist, 3.9% with LVEF <50% were either on GDMT or intolerant and 23.3% had other reasons for exclusion (Figure 1B).
Discussion
These findings suggest that RBS may be a useful tool for modeling and applying guideline logic in comparison to the traditional method of querying institutional health record repositories. While the RBS demonstrated acceptable performance in mass screening for remote GDMT optimization program, future efforts should focus on improving coding and integrating the natural language processing tool for processing of clinical notes to enhance screening accuracy and efficiency.
- Hassan, Shahzad ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Collins, Emma ( Mass General Brigham , Somerville , Massachusetts , United States )
- Figueroa, Christian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Ruggiero, Ryan ( Mass General Brigham , Somerville , Massachusetts , United States )
- Fridley, Echo ( Mass General Brigham , Somerville , Massachusetts , United States )
- Varugheese, Matthew ( Mass General Brigham , Somerville , Massachusetts , United States )
- Gabovitch, Dan ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Cannon, Christopher ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Desai, Akshay ( BRIGHAM WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Blood, Alexander ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Scirica, Benjamin ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Subramaniam, Samantha ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Wagholikar, Kavishwar ( Mass General Brigham , Somerville , Massachusetts , United States )
- Kumar, Sanjay ( Mass General Brigham , Somerville , Massachusetts , United States )
- Unlu, Ozan ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Zelle, David ( Mass General Brigham , Somerville , Massachusetts , United States )
- Ostrominski, John ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Nichols, Hunter ( Mass General Brigham , Somerville , Massachusetts , United States )
- Mcpartlin, Marian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Twinning, Megan ( Mass General Brigham , Somerville , Massachusetts , United States )
Meeting Info:
Session Info:
CardioTech Unleashed: Advances in Cardiovascular Diagnosis and Management
Monday, 11/18/2024 , 01:30PM - 02:30PM
Abstract Poster Session
More abstracts on this topic:
Shoemaker Camella, Shugrue Leah, Coccia Michael, Bakradze Ekaterina, Shapshak Dag, Gropen Toby, Thompson Karen, Taylor Danielle, Morrison Amanda, Stallings Ashley, Shipley Sarah, Jones Tamicka, Rafferty Rachael, Reid Tonya
AI-enabled ECG for Structural Heart Disease Diagnosis Improves Triage of Echocardiography Referral in a Low-Resource Setting: The PROVAR+ StudyPedroso Aline, Khera Rohan, Dhingra Lovedeep, Nascimento Bruno, Vasisht Shankar Sumukh, Sable Craig, Brant Luisa, Paixao Gabriela, Oliveira Clara, Ribeiro Antonio
More abstracts from these authors:
Unlu Ozan, Oates Michael, Cannon Christopher, Scirica Benjamin, Wagholikar Kavishwar, Aronson Samuel, Blood Alexander, Varugheese Matthew, Shin Jiyeon, Subramaniam Samantha, Stein David, St.laurent John, Mailly Charlotte, Mcpartlin Marian, Wang Fei
Main Results of the Cooperative Program for ImpLementation of Optimal Therapy in Heart Failure (COPILOT-HF) Program – A Pragmatic Randomized StudyBlood Alexander, Mcpartlin Marian, Wagholikar Kavishwar, Caberwal Harjeet, Cannon Christopher, Desai Akshay, Scirica Benjamin, Unlu Ozan, Hassan Shahzad, Nichols Hunter, Ostrominski John, Subramaniam Samantha, Zelle David, Figueroa Christian, Varugheese Matthew