Final ID: MP2233
Effects of Finerenone on Heart Failure Outcomes According to Baseline Heart Failure Risk: Insights from the FIDELITY Program
Introduction: The non-steroidal mineralocorticoid receptor antagonist finerenone reduces the risk of heart failure (HF) hospitalization in people with type 2 diabetes and chronic kidney disease (CKD), but whether these benefits vary according to baseline HF risk is uncertain.
Purpose: To evaluate the relative and absolute effects of finerenone on HF outcomes across different levels of HF risk, based on the Thrombolysis in Myocardial Infarction Risk Score for Heart Failure in Diabetes (TRS-HFDM).
Methods: We conducted a post-hoc individual participant data analysis of the FIDELITY program, consisting of the FIDELIO-DKD and FIGARO-DKD trials, which assessed the efficacy and safety of finerenone versus placebo on clinical outcomes in people with type 2 diabetes and albuminuric CKD. TRS-HFDM is a validated integer-based score (0-7 points) to predict HF events based on 5 clinical variables (history of HF [2 points], history of atrial fibrillation, history of coronary artery disease, eGFR <60 mL/min/1.73m2, UACR 30-300 mg/g, UACR >300 mg/g [2 points]). Participants were categorized into 4 groups according to TRS-HFDM score at baseline (0-1, 2, 3, 4+). Relative and absolute treatment effects on HF hospitalization or cardiovascular death were examined across TRS-HFDM score categories.
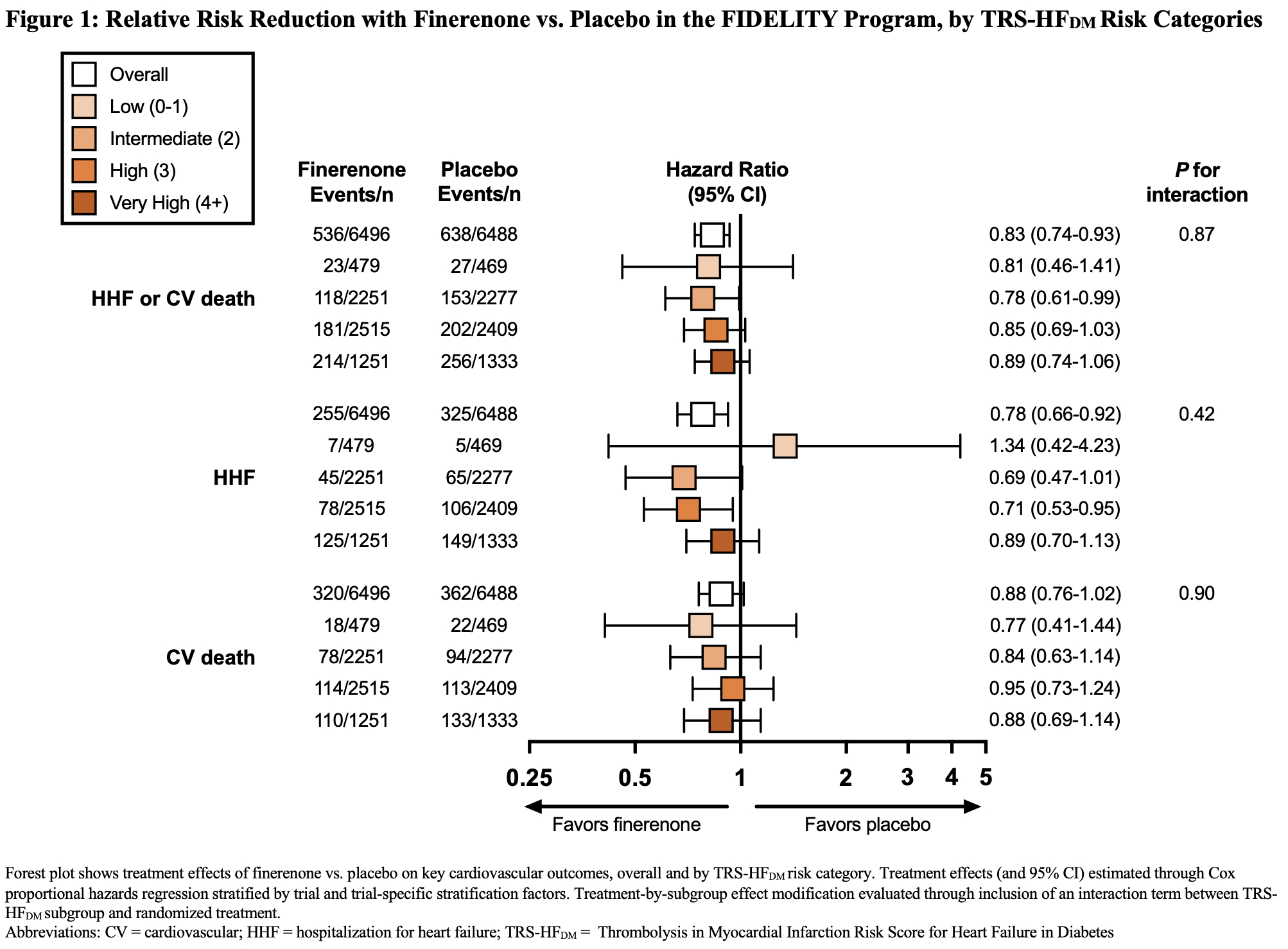
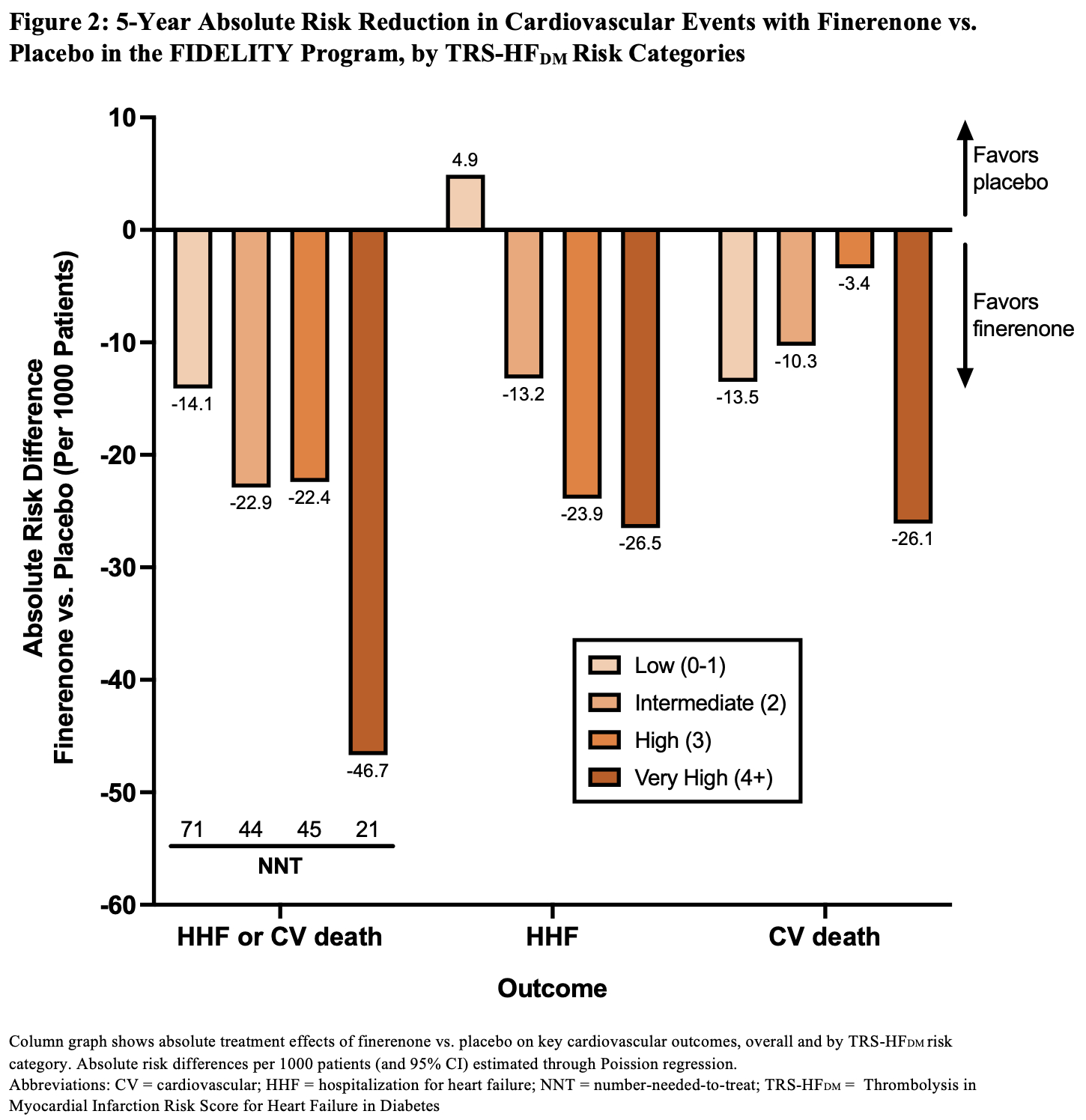
Results: Of 12,990 participants, 12,984 (99.95%) had available data to calculate TRS-HFDM. The proportion of participants who had a score of 0-1, 2, 3, and 4+, were 7.3%, 34.9%, 37.9%, and 19.9%, respectively. Compared with placebo, finerenone reduced the risk of time-to-first HF hospitalization or cardiovascular death (HR, 0.83; 95% CI, 0.74-0.93) with consistent relative effect across TRS-HFDM risk categories (Pinteraction=0.89; Figure 1). Relative effects on HF hospitalization (HR, 0.78; 95% CI, 0.66-0.92) and cardiovascular death (HR, 0.88; 95% CI, 0.76-1.02) were similarly consistent across TRS-HFDM risk categories (Pinteraction=0.42 and 0.90, respectively; Figure 1). Absolute risk reductions for HF hospitalization or cardiovascular death appeared greater across higher TRS-HFDM risk categories with fewer corresponding numbers needed to treat to prevent one outcome (Figure 2).
Conclusions: In people with type 2 diabetes and CKD, finerenone reduces the risk of HF hospitalization or cardiovascular death, with greater absolute benefits in people at higher risk for HF events.
- Ostrominski, John ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Brinker, Meike ( Bayer AG , Sao Paulo SP , Brazil )
- Schloemer, Patrick ( Bayer AG , Berlin , Germany )
- Rohwedder, Katja ( Bayer AG , Berlin , Germany )
- Mcmurray, John ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Vaduganathan, Muthiah ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Neuen, Brendon ( George Institute for Global Health , Newtown , New South Wales , Australia )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Agarwal, Rajiv ( VA Indianapolis , INDIANAPOLIS , Indiana , United States )
- Anker, Stefan ( charite , Berlin , Germany )
- Filippatos, Gerasimos ( NKUA , Chaidari , Greece )
- Rossing, Peter ( Steno Diabetes Center Copenhagen , Gentofte , Denmark )
- Ruilope, Luis ( Hospital 12 de Octubre , Madrid , Spain )
- Amarante, Flaviana ( Bayer AG , Sao Paulo SP , Brazil )
Meeting Info:
Session Info:
Heart Failure in CKM Syndrome: Prevention, Management and Outcomes
Monday, 11/10/2025 , 01:45PM - 02:55PM
Moderated Digital Poster Session
More abstracts on this topic:
Dabdoub Jorge, Wilson Michael, Gottbrecht Matthew, Salazar Ryan, Shih Jeffrey
ACTIVATION AND TARGETABILITY OF TYMP-IL-6-TF AXIS IN THE SKIN MICROENVIRONMENT IN UREMIC CALCIPHYLAXISLotfollahzadeh Saran, Chitalia Vipul
More abstracts from these authors:
Chatur Safia, Bakris George, Brinker Meike, Scalise Andrea, Schloemer Patrick, Rohwedder Katja, Solomon Scott, Filippatos Gerasimos, Vaduganathan Muthiah, Claggett Brian, Neuen Brendon, Pitt Bertram, Anker Stefan, Rossing Peter, Joseph Amer, Ruilope Luis
Estimated Lifetime Benefits of Finerenone on Cardiorenal Morbidity and Mortality in Patients with Type 2 Diabetes and Chronic Kidney DiseaseOstrominski John, Schloemer Patrick, Rohwedder Katja, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Anker Stefan, Neuen Brendon, Claggett Brian, Agarwal Rajiv, Filippatos Gerasimos, Rossing Peter, Ruilope Luis, Brinker Meike, Lage Andrea