Final ID: 4369294
Estimated Lifetime Benefits of Finerenone on Cardiorenal Morbidity and Mortality in Patients with Type 2 Diabetes and Chronic Kidney Disease
Introduction: Finerenone, a non-steroidal mineralocorticoid receptor antagonist, improves cardiovascular and kidney outcomes in people with type 2 diabetes and chronic kidney disease (CKD). However, the long-term impact of finerenone on delaying or preventing cardiorenal events is not known.
Purpose: To estimate the lifetime benefits of finerenone vs. placebo on cardiorenal morbidity and mortality in persons with type 2 diabetes and CKD.
Methods: In this participant-level pooled analysis of the FIDELIO-DKD and FIGARO-DKD trials (FIDELITY), we estimated the effects of finerenone on long-term survival free from cardiorenal morbidity and mortality using validated, nonparametric, and age-based actuarial methods. The main outcome was a cardiorenal composite outcome comprised of all-cause death and the individual components of the prespecified cardiovascular (heart failure hospitalization, non-fatal myocardial infarction, or non-fatal stroke) and kidney (CKD progression [sustained ≥57% decrease in eGFR] or kidney failure) composite endpoints.
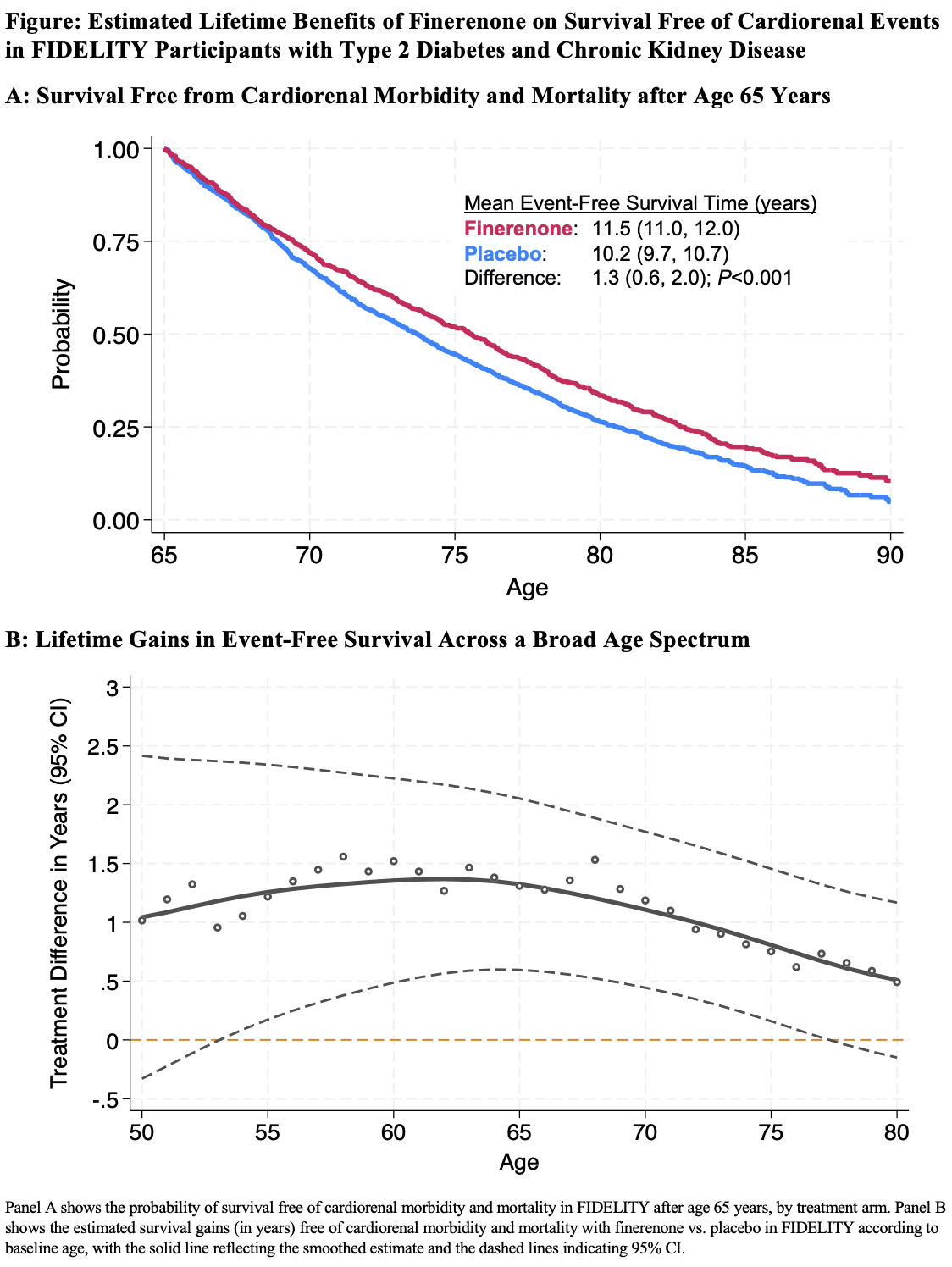
Results: Among 12,990 pooled trial participants, the risk of cardiorenal morbidity or mortality was lower with finerenone (71.8 events per 1000 person-years) vs. placebo (85.4 events per 1000 patient years; HR, 0.84 [95% CI, 0.78 to 0.90]) over a median trial follow-up of 3.0 [2.3, 3.8] years. In actuarial analyses examining lifetime trajectories for a 65-year-old trial participant, mean survival free from cardiorenal morbidity or mortality was 10.2 years (95% CI, 9.7 to 10.7) with placebo and 11.5 years (95% CI, 11.0 to 12.0) with finerenone, representing an additional 1.3 years (95% CI, 0.6 to 2.0 years) of event-free survival (Figure, Panel A). Cardiorenal event-free survival gains were observed across a broad age range, from 1.2 years (95% CI, 0.2 to 2.3 years) at age 55 to 0.8 years (95% CI, 0.1 to 1.4 years) at age 75 (Figure, Panel B).
Conclusions: Treatment with finerenone is projected to afford clinically relevant long-term gains in survival free from cardiorenal morbidity and mortality, reinforcing its role as a key component of guideline-directed medical therapy for persons with type 2 diabetes and CKD.
- Ostrominski, John ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Schloemer, Patrick ( Bayer AG , Berlin , Germany )
- Rohwedder, Katja ( Bayer AG , Berlin , Germany )
- Mcmurray, John ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Vaduganathan, Muthiah ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Anker, Stefan ( charite , Berlin , Germany )
- Neuen, Brendon ( George Institute for Global Health , Newtown , New South Wales , Australia )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Agarwal, Rajiv ( VA Indianapolis , INDIANAPOLIS , Indiana , United States )
- Filippatos, Gerasimos ( NKUA , Chaidari , Greece )
- Rossing, Peter ( Steno Diabetes Center Copenhagen , Gentofte , Denmark )
- Ruilope, Luis ( Hospital 12 de Octubre , Madrid , Spain )
- Brinker, Meike ( Bayer AG , Berlin , Germany )
- Lage, Andrea ( Bayer SA , Sao Paulo , Brazil )
Meeting Info:
Session Info:
Refining Therapeutic Approaches for CKM Syndrome
Saturday, 11/08/2025 , 01:30PM - 02:35PM
Abstract Oral Session
More abstracts on this topic:
Madhusudhan Divya, Pressley Alyssa, El Sayed Nuha, Okeke Oge, Bradley Sarah, Blanco Caroline, Perla Esteban, Jennings Ruby, Picou Kylie, Mcweeny Patrick, Crabill Carrianne
Angiopoeitin-2 and Mortality in an End-Stage Renal Disease, Heart Failure PopulationRobbin Vanessa, Bansal Vinod, Siddiqui Fakiha, Fareed Jawed, Syed Mushabbar
More abstracts from these authors:
Chatur Safia, Bakris George, Brinker Meike, Scalise Andrea, Schloemer Patrick, Rohwedder Katja, Solomon Scott, Filippatos Gerasimos, Vaduganathan Muthiah, Claggett Brian, Neuen Brendon, Pitt Bertram, Anker Stefan, Rossing Peter, Joseph Amer, Ruilope Luis
Effects of Finerenone on Heart Failure Outcomes According to Baseline Heart Failure Risk: Insights from the FIDELITY ProgramOstrominski John, Brinker Meike, Schloemer Patrick, Rohwedder Katja, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Neuen Brendon, Claggett Brian, Agarwal Rajiv, Anker Stefan, Filippatos Gerasimos, Rossing Peter, Ruilope Luis, Amarante Flaviana