Final ID: MP452
Finerenone Reduces Loop Diuretic Requirement in Patients with Type 2 Diabetes and CKD: Participant-Level Pooled Analysis of FIDELIO-DKD and FIGARO-DKD
Methods: This was a post hoc analysis of FIDELITY, a participant-level pooled analysis of the FIDELIO-DKD and FIGARO-DKD trials, which enrolled patients with T2D and CKD. We evaluated the association between the need for new loop diuretic (LD) initiation and rates of subsequent all-cause mortality. We then assessed the treatment effect of finerenone relative to placebo on new LD initiation among diuretic-naïve individuals overall and according to baseline eGFR and UACR. We finally examined the effects of finerenone on an expanded composite CV endpoint adding new diuretic initiation to the prespecified FIDELITY endpoint of CV death, non-fatal MI, non-fatal stroke, and hospitalization for HF.
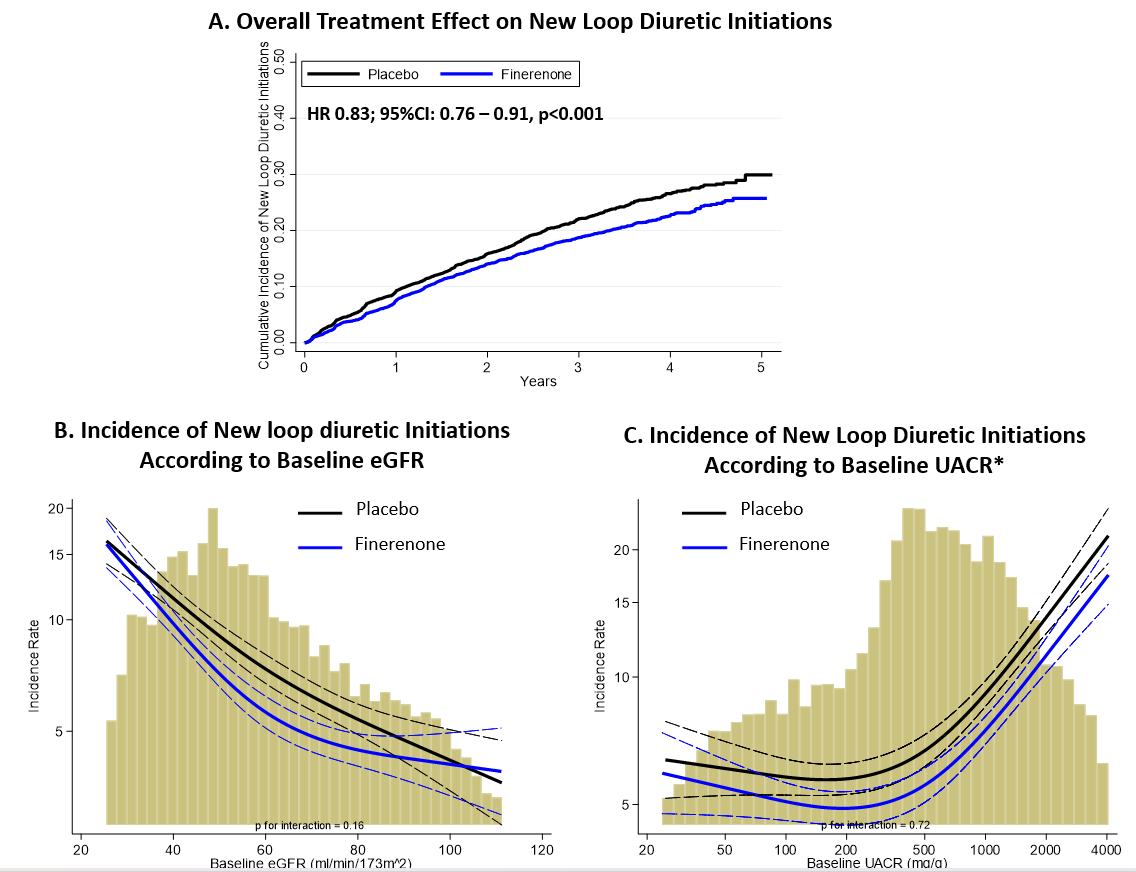
Results: Among 12, 990 participants in FIDELITY, 10,194 (78%) were not treated with a LD at baseline and new diuretic initiation occurred in 2,107(22%) in follow up. New LD requirement was more frequent among those with lower eGFR and higher UACR at baseline (Figure 1). Patients requiring new diuretic initiation experienced rates of subsequent mortality that were nearly quadruple (8.8 [7.8-9.9] per 100 patient-years) that of those not requiring diuretic initiation (1.9 [1.7-2.0] per 100 patient-years). Treatment with finerenone reduced new initiations of LDs by 17% (HR 0.83; 95% CI: 0.76-0.91, p<0.001) in FIDELITY (Figure 1A). Relative to placebo, the reduction in the incidence of new LD initiations with finerenone was also consistent across the spectrum of both eGFR (Figure 1B) and UACR (Figure 1C). Adding new LD initiation to the prespecified FIDELITY CV composite endpoint nearly doubled the number of events from 1,764 to 3,286 and underscored the cardiovascular benefits of finerenone (HR 0.85; 95% CI: 0.80-0.91, p<0.001).
Conclusions: In patients with T2D and CKD, new requirement for diuretic initiation occurred in ~1 in 5 patients and was associated with higher rates of subsequent mortality. Treatment with finerenone significantly reduced new LD initiation, an effect that was consistent across the kidney function spectrum. These data highlight the diuretic-sparing potential of finerenone in patients with T2D and CKD.
- Chatur, Safia ( Brigham and Women`s Hospital/Harvard , Boston , Massachusetts , United States )
- Bakris, George ( Rush University , Chicagor , Illinois , United States )
- Brinker, Meike ( Bayer AG , Berlin , Germany )
- Scalise, Andrea ( Bayer AG , Berlin , Germany )
- Schloemer, Patrick ( Bayer AG , Berlin , Germany )
- Rohwedder, Katja ( Bayer AG , Berlin , Germany )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Filippatos, Gerasimos ( NKUA , Chaidari , Greece )
- Vaduganathan, Muthiah ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Neuen, Brendon ( George Institute for Global Health , Newtown , New South Wales , Australia )
- Pitt, Bertram ( University of Michigan School , Ann Arbor , Michigan , United States )
- Anker, Stefan ( charite , Berlin , Germany )
- Rossing, Peter ( Steno Diabetes Center Copenhagen , Gentofte , Denmark )
- Joseph, Amer ( Bayer , Berlin , Germany )
- Ruilope, Luis ( Hospital 12 de Octubre , Madrid , Spain )
Meeting Info:
Session Info:
Addressing the Cardiac-Kidney Connection: Defining Risk and Optimizing Outcomes
Saturday, 11/08/2025 , 01:45PM - 02:45PM
Moderated Digital Poster Session
More abstracts on this topic:
Mirza Imaduddin, Morsy Mohammed, Levitan Irena, Raj Usha, Mahmoud Abeer
37,500 man-years of clinical expertise validate the cardio-metabolic benefits of empagliflozin-linagliptin in type 2 diabetes: findings from the amplified consensusNair Tiny, Jabbar P, Ray Saumitra, Hazra Prakash, Gupta Sushil, Joshi Ameya, Shaikh Shehla, B Jayagopal, Pandit Kaushik, Sharma D, Seshadri Krishna, S Sridhar
More abstracts from these authors:
Ostrominski John, Schloemer Patrick, Rohwedder Katja, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Anker Stefan, Neuen Brendon, Claggett Brian, Agarwal Rajiv, Filippatos Gerasimos, Rossing Peter, Ruilope Luis, Brinker Meike, Lage Andrea
Effects of Finerenone on Heart Failure Outcomes According to Baseline Heart Failure Risk: Insights from the FIDELITY ProgramOstrominski John, Brinker Meike, Schloemer Patrick, Rohwedder Katja, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Neuen Brendon, Claggett Brian, Agarwal Rajiv, Anker Stefan, Filippatos Gerasimos, Rossing Peter, Ruilope Luis, Amarante Flaviana