Final ID: MP2770
Preservation of Acetyl-Coenzyme A Synthetase 2 Sustains Cytosolic Acetyl-CoA and Protects Against Heart Failure
Abstract Body (Do not enter title and authors here): Background:
Emerging evidence suggests that the homeostasis of cytosolic acetyl-coenzyme A (acetyl-CoA) is critical for maintaining cardiac function, especially under pathological stress. Acetyl-CoA serves as a central metabolic intermediate, linking nutrient catabolism to mitochondrial energy production and epigenetic regulation through protein acetylation. However, the enzymatic pathways that control cytosolic acetyl-CoA levels in the heart remain poorly defined. Acetyl-CoA synthetase 2 (ACSS2), which converts acetate into acetyl-CoA in the cytosol, may play a key role in regulating this metabolic pool. This study aimed to investigate the role of ACSS2 in cardiomyocyte metabolism and adaptation in response to isoproterenol.
Methods:
In vivo, mice were infused with isoproterenol via osmotic pump to induce heart failure, and cardiac function was assessed using echocardiography. In vitro, H9c2 cardiomyoblasts were treated with isoproterenol and subjected to either pharmacological inhibition or CRISPR/Cas9-mediated knockout of ACSS2. Cytosolic acetyl-CoA and mitochondrial respiration were measured using targeted metabolite assays and seahorse XF analysis. ACSS2 was overexpressed using lentiviral vectors. Short- and medium-chain fatty acids, specifically butyrate and octanoate, were supplemented to bypass mitochondrial transport steps and assess mitochondrial function.
Results:
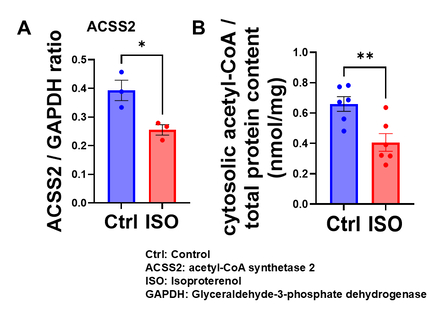
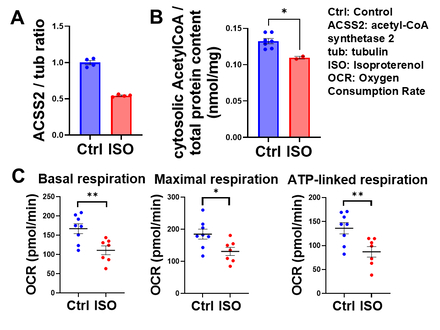
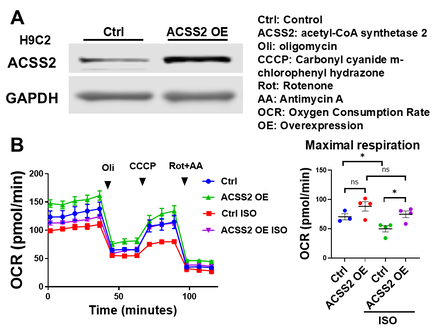
Western blot analysis showed that ACSS2 expression was significantly reduced in the myocardium of isoproterenol-treated mice (Fig. 1A), with a corresponding decrease in cytosolic acetyl-CoA levels (Fig. 1B). In H9c2 cells, isoproterenol similarly reduced ACSS2 expression (Fig. 2A) and cytosolic acetyl-CoA (Fig. 2B). Isoproterenol impaired mitochondrial respiration, with reductions in basal, maximal, and ATP-linked respiration (Fig. 2C). Both pharmacological inhibition and genetic deletion of ACSS2 impaired mitochondrial respiration. In contrast, ACSS2 overexpression preserved mitochondrial function under isoproterenol stress (Fig. 3). Supplementation with butyrate or octanoate partially improved mitochondrial respiration, but did not fully rescue mitochondrial function, suggesting that cytosolic acetyl-CoA depletion causes mitochondrial dysfunction beyond substrate limitation.
Conclusions:
ACSS2 is essential for maintaining cytosolic acetyl-CoA levels and mitochondrial function during cardiac stress. These findings support acetate metabolism as a therapeutic target for heart failure.
Emerging evidence suggests that the homeostasis of cytosolic acetyl-coenzyme A (acetyl-CoA) is critical for maintaining cardiac function, especially under pathological stress. Acetyl-CoA serves as a central metabolic intermediate, linking nutrient catabolism to mitochondrial energy production and epigenetic regulation through protein acetylation. However, the enzymatic pathways that control cytosolic acetyl-CoA levels in the heart remain poorly defined. Acetyl-CoA synthetase 2 (ACSS2), which converts acetate into acetyl-CoA in the cytosol, may play a key role in regulating this metabolic pool. This study aimed to investigate the role of ACSS2 in cardiomyocyte metabolism and adaptation in response to isoproterenol.
Methods:
In vivo, mice were infused with isoproterenol via osmotic pump to induce heart failure, and cardiac function was assessed using echocardiography. In vitro, H9c2 cardiomyoblasts were treated with isoproterenol and subjected to either pharmacological inhibition or CRISPR/Cas9-mediated knockout of ACSS2. Cytosolic acetyl-CoA and mitochondrial respiration were measured using targeted metabolite assays and seahorse XF analysis. ACSS2 was overexpressed using lentiviral vectors. Short- and medium-chain fatty acids, specifically butyrate and octanoate, were supplemented to bypass mitochondrial transport steps and assess mitochondrial function.
Results:
Western blot analysis showed that ACSS2 expression was significantly reduced in the myocardium of isoproterenol-treated mice (Fig. 1A), with a corresponding decrease in cytosolic acetyl-CoA levels (Fig. 1B). In H9c2 cells, isoproterenol similarly reduced ACSS2 expression (Fig. 2A) and cytosolic acetyl-CoA (Fig. 2B). Isoproterenol impaired mitochondrial respiration, with reductions in basal, maximal, and ATP-linked respiration (Fig. 2C). Both pharmacological inhibition and genetic deletion of ACSS2 impaired mitochondrial respiration. In contrast, ACSS2 overexpression preserved mitochondrial function under isoproterenol stress (Fig. 3). Supplementation with butyrate or octanoate partially improved mitochondrial respiration, but did not fully rescue mitochondrial function, suggesting that cytosolic acetyl-CoA depletion causes mitochondrial dysfunction beyond substrate limitation.
Conclusions:
ACSS2 is essential for maintaining cytosolic acetyl-CoA levels and mitochondrial function during cardiac stress. These findings support acetate metabolism as a therapeutic target for heart failure.
More abstracts on this topic:
Alfa-tubulin detyrosination causes mitochondrial dysfunction through suppression of Parkin-mediated mitophagy linking to heart failure with preserved ejection fraction
Miura Shunsuke, Nakazato Kazuhiko, Ishida Takafumi, Takeishi Yasuchika, Sekine Toranosuke, Ogawara Ryo, Ichimura Shohei, Yokokawa Tetsuro, Misaka Tomofumi, Oikawa Masayoshi, Kobayashi Atsushi, Yamaki Takayoshi
A Case of Steroid-Refractory Immune-checkpoint-inhibitor Induced Myocarditis Responsive to Mycophenolate and Anti-thymocyte globulinDabdoub Jorge, Wilson Michael, Gottbrecht Matthew, Salazar Ryan, Shih Jeffrey