Final ID: MP2775
Exacerbation of Doxorubicin-Induced Mitochondrial Dysfunction by the Inhibition of Voltage -Dependent Anion Channel 1 Oligomerization
Abstract Body (Do not enter title and authors here): Background:
Doxorubicin-induced cardiomyopathy is a widely used model of heart failure associated with mitochondrial dysfunction. Voltage-dependent anion channel 1 (VDAC1) oligomerization contributes to mitochondrial membrane permeabilization and cell death. Although inhibition of VDAC1 oligomerization has shown cytoprotective effects in some disease contexts, its role in doxorubicin-induced cardiac injury remains unclear, particularly in relation to mitochondrial iron homeostasis and oxidative stress.
Methods:
H9c2 cardiomyoblasts were treated with doxorubicin (DOX), the VDAC1 oligomerization inhibitor VBIT-4, or both. Mitochondrial respiration was assessed using the Seahorse XF analyzer. Cell viability was evaluated via propidium iodide (PI) and Hoechst staining. Mitochondrial iron levels and reactive oxygen species (ROS) were measured using Mito-FerroGreen and MitoSOX Red, respectively. To investigate the role of VDAC1 oligomerization, H9c2 cells were engineered to overexpress either wild-type VDAC1 or an oligomerization-deficient mutant (VDAC1 K53R/K274R), in which key lysine residues required for ubiquitin-mediated oligomerization were replaced with arginine.
Results:
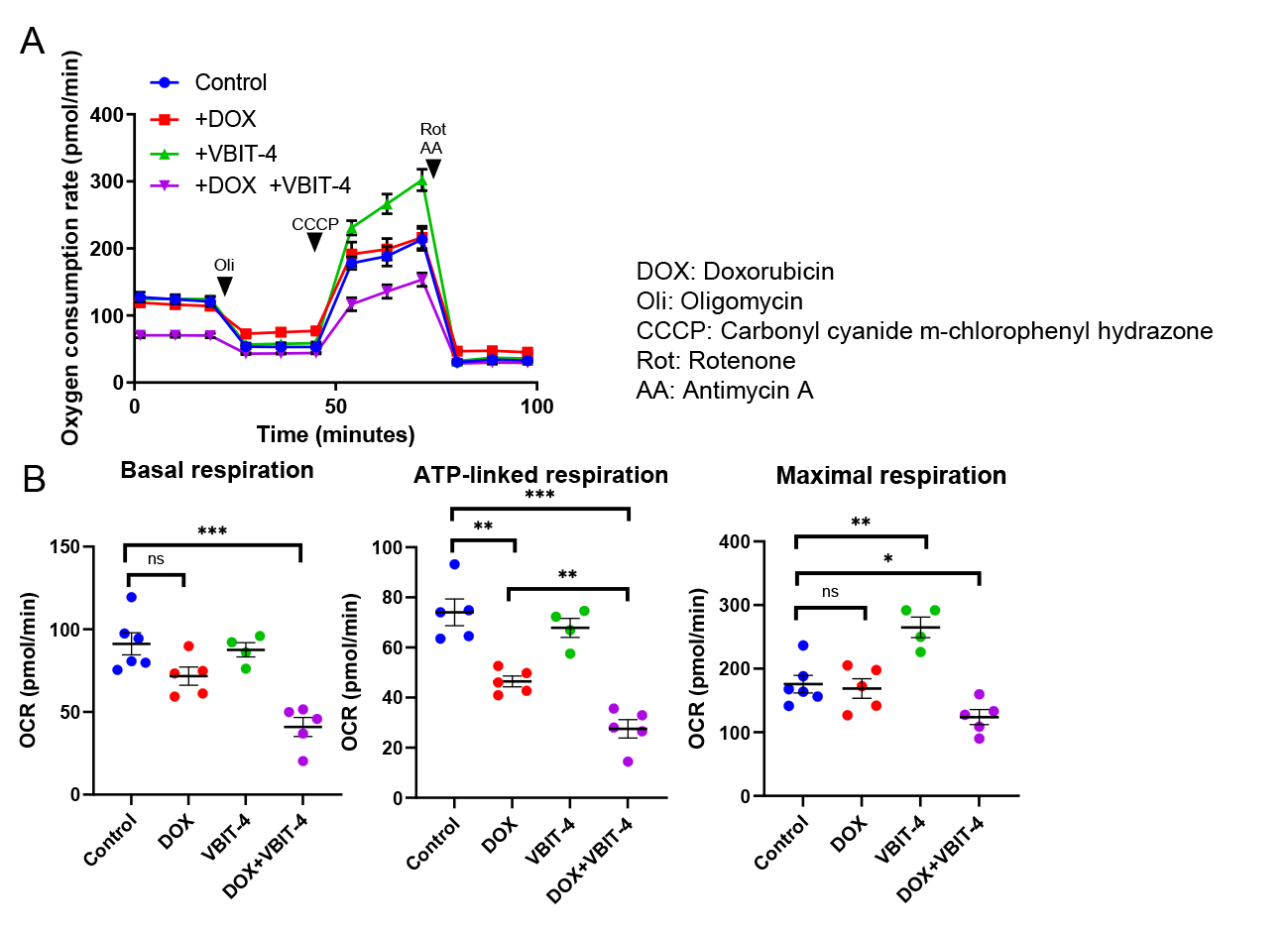
VBIT-4 alone did not affect mitochondrial respiration or cell viability. However, co-treatment with DOX and VBIT-4 resulted in a significantly greater reduction in oxygen consumption rate (OCR) than DOX alone (Fig. 1A, B). PI and Hoechst staining indicated increased cell death under co-treatment.Confocal microscopy revealed that co-treatment with DOX and VBIT-4 significantly increased mitochondrial iron accumulation and ROS production compared to DOX treatment alone (Fig. 2A, B), suggesting an exacerbation of iron-driven oxidative damage. Moreover, VDAC1 K53R/K274R-overexpressing cells were more susceptible to DOX-induced mitochondrial dysfunction, showing greater reductions in basal, ATP-linked, and maximal OCRs compared to wild-type VDAC1-overexpressing cells (Fig. 3A, B), along with elevated Mito-FerroGreen and MitoSOX fluorescence.
Conclusions:
Unexpectedly, the inhibition of VDAC1 oligomerization exacerbated doxorubicin-induced mitochondrial dysfunction, likely through increased mitochondrial iron accumulation and oxidative stress. These findings suggest a potentially protective, context-dependent role for VDAC1 oligomerization in cardiomyocytes and call for careful consideration when targeting VDAC1 in cardiotoxic conditions.
Doxorubicin-induced cardiomyopathy is a widely used model of heart failure associated with mitochondrial dysfunction. Voltage-dependent anion channel 1 (VDAC1) oligomerization contributes to mitochondrial membrane permeabilization and cell death. Although inhibition of VDAC1 oligomerization has shown cytoprotective effects in some disease contexts, its role in doxorubicin-induced cardiac injury remains unclear, particularly in relation to mitochondrial iron homeostasis and oxidative stress.
Methods:
H9c2 cardiomyoblasts were treated with doxorubicin (DOX), the VDAC1 oligomerization inhibitor VBIT-4, or both. Mitochondrial respiration was assessed using the Seahorse XF analyzer. Cell viability was evaluated via propidium iodide (PI) and Hoechst staining. Mitochondrial iron levels and reactive oxygen species (ROS) were measured using Mito-FerroGreen and MitoSOX Red, respectively. To investigate the role of VDAC1 oligomerization, H9c2 cells were engineered to overexpress either wild-type VDAC1 or an oligomerization-deficient mutant (VDAC1 K53R/K274R), in which key lysine residues required for ubiquitin-mediated oligomerization were replaced with arginine.
Results:
VBIT-4 alone did not affect mitochondrial respiration or cell viability. However, co-treatment with DOX and VBIT-4 resulted in a significantly greater reduction in oxygen consumption rate (OCR) than DOX alone (Fig. 1A, B). PI and Hoechst staining indicated increased cell death under co-treatment.Confocal microscopy revealed that co-treatment with DOX and VBIT-4 significantly increased mitochondrial iron accumulation and ROS production compared to DOX treatment alone (Fig. 2A, B), suggesting an exacerbation of iron-driven oxidative damage. Moreover, VDAC1 K53R/K274R-overexpressing cells were more susceptible to DOX-induced mitochondrial dysfunction, showing greater reductions in basal, ATP-linked, and maximal OCRs compared to wild-type VDAC1-overexpressing cells (Fig. 3A, B), along with elevated Mito-FerroGreen and MitoSOX fluorescence.
Conclusions:
Unexpectedly, the inhibition of VDAC1 oligomerization exacerbated doxorubicin-induced mitochondrial dysfunction, likely through increased mitochondrial iron accumulation and oxidative stress. These findings suggest a potentially protective, context-dependent role for VDAC1 oligomerization in cardiomyocytes and call for careful consideration when targeting VDAC1 in cardiotoxic conditions.
More abstracts on this topic:
Chronic Oxidative Stress Induces Hypertension and Abdominal Aortic Aneurysm in Chemogenetic Mice Model
Das Apabrita, Waldeck-weiermair Markus, Yadav Shambhu, Covington Taylor, Spyropoulos Fotios, Pandey Arvind, Michel Thomas
Endotoxemia induces mitochondrial DNA (mtDNA) damage in mouse myocardium and cardiomyocytesDubey Praveen, Singh Sarojini, Meeler Avery, Krishnamurthy Prasanna