Final ID: MP1041
Patient-Reported Outcomes in Patients with Symptomatic, Obstructive Hypertrophic Cardiomyopathy Treated with Mavacamten: Real-world Observations through Week 30 from the COMPASS-HCM Study
Abstract Body (Do not enter title and authors here): Background: Clinical trials have demonstrated mavacamten’s efficacy in improving health status of patients with obstructive hypertrophic cardiomyopathy (oHCM), but real-world data remain limited.
Research Question: What is mavacamten’s impact on health status changes in patients with oHCM in real-world settings?
Methods: The COMPASS-HCM study (NCT06551129) prospectively recruited US adults with oHCM prescribed mavacamten, excluding those with moderate lung disease, recent hospitalization (≤ 2 weeks), recent heart/lung surgery or stroke/transient ischemic attack (≤ 6 months), or mavacamten treatment ≥7 days prior to enrollment. Participants completed the Kansas City Cardiomyopathy Questionnaire (KCCQ; higher scores are better) and HCM Symptom Questionnaire (HCMSQ; lower scores are better) at baseline, weeks 2, 4, 8, 12, 24, and 30 after initially starting mavacamten. Patient characteristics and health status changes from baseline through week 30 were described.
Results: A total of 108 patients (mean [± SD] age 66.7 ± 12.5 years, 61.1% women, and 94.4% White) completed baseline surveys and initiated mavacamten following enrollment. Baseline mean scores were 60.3 ± 20.9 for KCCQ Overall Summary Score (KCCQ-OSS), 65.9 ± 19.3 for KCCQ Clinical Summary Score (KCCQ-CSS), 6.9 ± 3.5 for HCMSQ Shortness of Breath (SoB) domain, and 4.5 ± 2.0 for HCMSQ Total score. At baseline, 85.2% of patients were on background oHCM therapy.
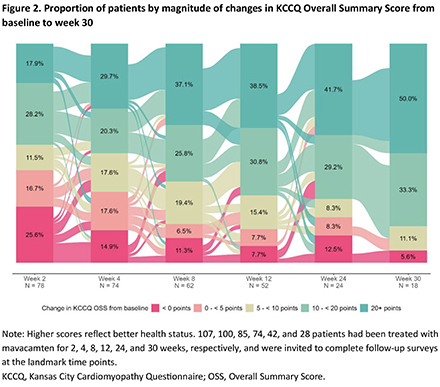
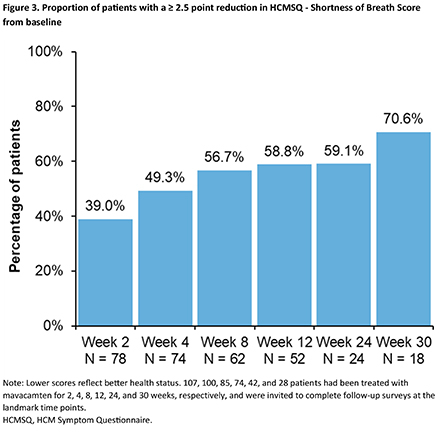
Follow-up surveys were completed by 78, 74, 62, 52, 24, and 18 patients treated with mavacamten for 2, 4, 8, 12, 24, and 30 weeks, respectively. At last follow-up, daily mavacamten dose was 2.5 mg in 16.7%, 5 mg in 38.9%, 10 mg in 22.2%, and 15 mg in 22.2% of patients. Mean improvements from baseline to week 30 were 20.7 ± 17.1 for KCCQ-OSS (Figure 1), 16.4 ± 12.8 for KCCQ-CSS, -3.9 ± 3.5 for HCMSQ SoB domain, and -2.0 ± 2.0 for HCMSQ Total score. At week 12, 69.2% and 38.5% of patients achieved large (≥10 points) and very large (≥20 points) improvements in KCCQ-OSS, respectively, increasing to 83.3% and 50.0% at week 30 (Figure 2). A ≥ 2.5 point reduction in HCMSQ-SoB score was observed in 58.8% of patients at week 12, increasing to 70.6% at week 30 (Figure 3).
Conclusion(s): Real-world mavacamten treatment led to rapid and substantial health status improvements that appear to accrue through week 30 in oHCM, highly consistent with the EXPLORER-HCM findings in a more restricted population.
Research Question: What is mavacamten’s impact on health status changes in patients with oHCM in real-world settings?
Methods: The COMPASS-HCM study (NCT06551129) prospectively recruited US adults with oHCM prescribed mavacamten, excluding those with moderate lung disease, recent hospitalization (≤ 2 weeks), recent heart/lung surgery or stroke/transient ischemic attack (≤ 6 months), or mavacamten treatment ≥7 days prior to enrollment. Participants completed the Kansas City Cardiomyopathy Questionnaire (KCCQ; higher scores are better) and HCM Symptom Questionnaire (HCMSQ; lower scores are better) at baseline, weeks 2, 4, 8, 12, 24, and 30 after initially starting mavacamten. Patient characteristics and health status changes from baseline through week 30 were described.
Results: A total of 108 patients (mean [± SD] age 66.7 ± 12.5 years, 61.1% women, and 94.4% White) completed baseline surveys and initiated mavacamten following enrollment. Baseline mean scores were 60.3 ± 20.9 for KCCQ Overall Summary Score (KCCQ-OSS), 65.9 ± 19.3 for KCCQ Clinical Summary Score (KCCQ-CSS), 6.9 ± 3.5 for HCMSQ Shortness of Breath (SoB) domain, and 4.5 ± 2.0 for HCMSQ Total score. At baseline, 85.2% of patients were on background oHCM therapy.
Follow-up surveys were completed by 78, 74, 62, 52, 24, and 18 patients treated with mavacamten for 2, 4, 8, 12, 24, and 30 weeks, respectively. At last follow-up, daily mavacamten dose was 2.5 mg in 16.7%, 5 mg in 38.9%, 10 mg in 22.2%, and 15 mg in 22.2% of patients. Mean improvements from baseline to week 30 were 20.7 ± 17.1 for KCCQ-OSS (Figure 1), 16.4 ± 12.8 for KCCQ-CSS, -3.9 ± 3.5 for HCMSQ SoB domain, and -2.0 ± 2.0 for HCMSQ Total score. At week 12, 69.2% and 38.5% of patients achieved large (≥10 points) and very large (≥20 points) improvements in KCCQ-OSS, respectively, increasing to 83.3% and 50.0% at week 30 (Figure 2). A ≥ 2.5 point reduction in HCMSQ-SoB score was observed in 58.8% of patients at week 12, increasing to 70.6% at week 30 (Figure 3).
Conclusion(s): Real-world mavacamten treatment led to rapid and substantial health status improvements that appear to accrue through week 30 in oHCM, highly consistent with the EXPLORER-HCM findings in a more restricted population.
More abstracts on this topic:
A Precision Medicine Approach to Predicting Pathogenicity and Disease Penetrance Among Variants in Hypertrophic Cardiomyopathy-Associated Genes at a Population Level
Wolfe Rachel, Chahal Anwar, Landstrom Andrew, Kurzlechner Leonie, Monaco Gabrielle, Yadav Kanishk, Gurumoorthi Manasa, Sharaf Dabbagh Ghaith, Shah Ravi, Williams Trevor, Facio Flavia
AI-enhanced Electrocardiographic Evaluation of Left Ventricular Ejection Fraction and Outflow Tract Gradient in Hypertrophic CardiomyopathySangha Veer, Aminorroaya Arya, Dhingra Lovedeep, Pedroso Aline, Oikonomou Evangelos, Khera Rohan