Final ID: Su2191
Real-world treatment patterns of mavacamten and associated background therapies in patients with obstructive hypertrophic cardiomyopathy (HCM) in the United States
Abstract Body (Do not enter title and authors here): Background: There are limited data on the treatment patterns of mavacamten and background therapies in real-world patients with obstructive HCM.
Aims: To describe posology, discontinuation, adherence to mavacamten, and the use of HCM background therapies in real-world patients.
Methods: This was a retrospective cohort study of adult patients using the Symphony Integrated Dataverse from Apr 28, 2022 to Jan 18, 2024. Patients were included if they had ≥ 1 approved claim for mavacamten and had continuous claims activity during the 12 months (baseline) before the first claim (index date). The stable dose (defined as the dose used for 6 months consecutively), discontinuation (defined as a treatment gap ≥ 95 days without restarting), and adherence (measured as proportion of days covered [PDC]) were described for mavacamten. The use of background therapies (beta blockers, calcium channel blockers and disopyramide) was described at index and during the follow-up.
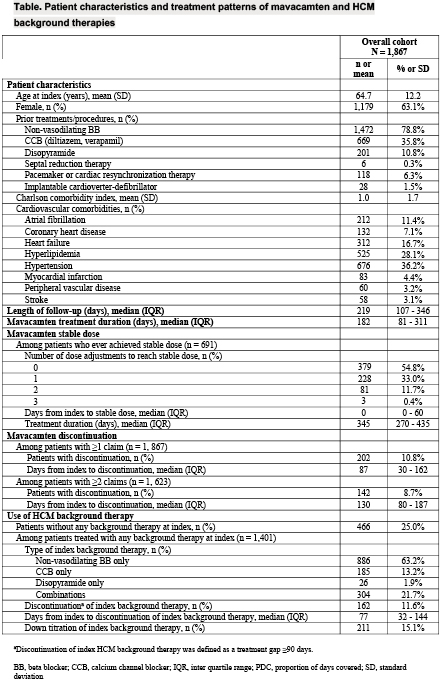
Results: A total of 1867 patients (mean ± SD age 64.7 ± 12.2 years, 63.1% female, median [interquartile range, IQR] follow-up 219 [107, 346] days) were included (Table). Among patients with ≥ 6 (n = 993), ≥ 9 (n = 640), and ≥12 (n = 394) months of treatment, 67.3%, 86.1%, and 93.7% reached stable dose, respectively. Among patients who reached stable dose (n = 691, median [IQR] treatment duration 345 [270, 435] days), 54.8% did not require any dose adjustment, and 33.0% required only one adjustment from treatment initiation. The most commonly used stable dose was 5 mg (49.6%), followed by 2.5 mg (25.0%), 10 mg (20.5%) and 15 mg (4.8%). The discontinuation rate for mavacamten was 10.8% and 8.7% among patients with ≥1 and ≥2 claims, respectively. Mean PDC for mavacamten was 93.7 ± 9.1%. Overall, 25% of patients did not have any claim for background therapy at index. For the remaining patients (n = 1401), 11.6% discontinued and 15.1% down titrated the index HCM background therapy.
Conclusions: The vast majority of patients treated with mavacamten reached stable dose with no or only one dose adjustment. Discontinuation rate was 8.7-10.8% and adherence to mavacamten was high. A substantial proportion of patients discontinued or down titrated HCM background therapy.
Aims: To describe posology, discontinuation, adherence to mavacamten, and the use of HCM background therapies in real-world patients.
Methods: This was a retrospective cohort study of adult patients using the Symphony Integrated Dataverse from Apr 28, 2022 to Jan 18, 2024. Patients were included if they had ≥ 1 approved claim for mavacamten and had continuous claims activity during the 12 months (baseline) before the first claim (index date). The stable dose (defined as the dose used for 6 months consecutively), discontinuation (defined as a treatment gap ≥ 95 days without restarting), and adherence (measured as proportion of days covered [PDC]) were described for mavacamten. The use of background therapies (beta blockers, calcium channel blockers and disopyramide) was described at index and during the follow-up.
Results: A total of 1867 patients (mean ± SD age 64.7 ± 12.2 years, 63.1% female, median [interquartile range, IQR] follow-up 219 [107, 346] days) were included (Table). Among patients with ≥ 6 (n = 993), ≥ 9 (n = 640), and ≥12 (n = 394) months of treatment, 67.3%, 86.1%, and 93.7% reached stable dose, respectively. Among patients who reached stable dose (n = 691, median [IQR] treatment duration 345 [270, 435] days), 54.8% did not require any dose adjustment, and 33.0% required only one adjustment from treatment initiation. The most commonly used stable dose was 5 mg (49.6%), followed by 2.5 mg (25.0%), 10 mg (20.5%) and 15 mg (4.8%). The discontinuation rate for mavacamten was 10.8% and 8.7% among patients with ≥1 and ≥2 claims, respectively. Mean PDC for mavacamten was 93.7 ± 9.1%. Overall, 25% of patients did not have any claim for background therapy at index. For the remaining patients (n = 1401), 11.6% discontinued and 15.1% down titrated the index HCM background therapy.
Conclusions: The vast majority of patients treated with mavacamten reached stable dose with no or only one dose adjustment. Discontinuation rate was 8.7-10.8% and adherence to mavacamten was high. A substantial proportion of patients discontinued or down titrated HCM background therapy.
More abstracts on this topic:
Artificial Intelligence-based screening for Hypertrophic Cardiomyopathy from Single-lead Electrocardiograms: A Multinational Development and Validation Study
Croon Philip, Aminorroaya Arya, Pedroso Aline, Dhingra Lovedeep, Khera Rohan
A Multicenter Friedreich Ataxia Registry Identifies Posterior Wall Thickness as a Predictor of Major Adverse Cardiac EventsLin Kimberly, Johnson Jonathan, Mccormack Shana, Lynch David, Tate Barbara, Feng Yixuan, Huang Jing, Mercer-rosa Laura, Dedio Anna, Mcsweeney Kara, Fournier Anne, Yoon Grace, Payne Ronald, Cripe Linda, Patel Aarti, Niaz Talha