Final ID: MP1922
Artificial Intelligence-based screening for Hypertrophic Cardiomyopathy from Single-lead Electrocardiograms: A Multinational Development and Validation Study
Abstract Body (Do not enter title and authors here): Background: Hypertrophic cardiomyopathy (HCM) is a leading cause of sudden death in young and middle-aged adults and frequently goes undiagnosed due to the absence of accessible and scalable screening strategies. Artificial intelligence (AI) applied to single-lead electrocardiograms (ECGs) from portable devices offers a promising approach for large-scale screening. However, noisy signals can substantially compromise diagnostic accuracy.
Aim: We developed and validated a noise-adapted AI-ECG model specifically designed to detect HCM from noisy single-lead ECGs.
Methods: We developed an AI-ECG model using lead I from 160,396 unique 12-lead ECGs of 85,967 individuals in the Yale New Haven Health System (YNHHS) and augmented the ECG signal with real-world noises to develop noise-resilient models. A held-out test including 38,426 ECGs from unique individuals (mean age of 53.9 ± 19.3 years, 20,309 [53%] women) was used for internal validation. There were 59 (0.2%) HCM cases, adjudicated by expert clinicians using cardiac magnetic resonance (CMR) imaging. External validation was performed in manually validated MIMIC-IV (n=995, 66 HCM cases) and the UK Biobank (n=57,963, 53 HCM cases). To assess model fairness, we conducted stratified analyses by age, sex, race/ethnicity, and key ECG features.
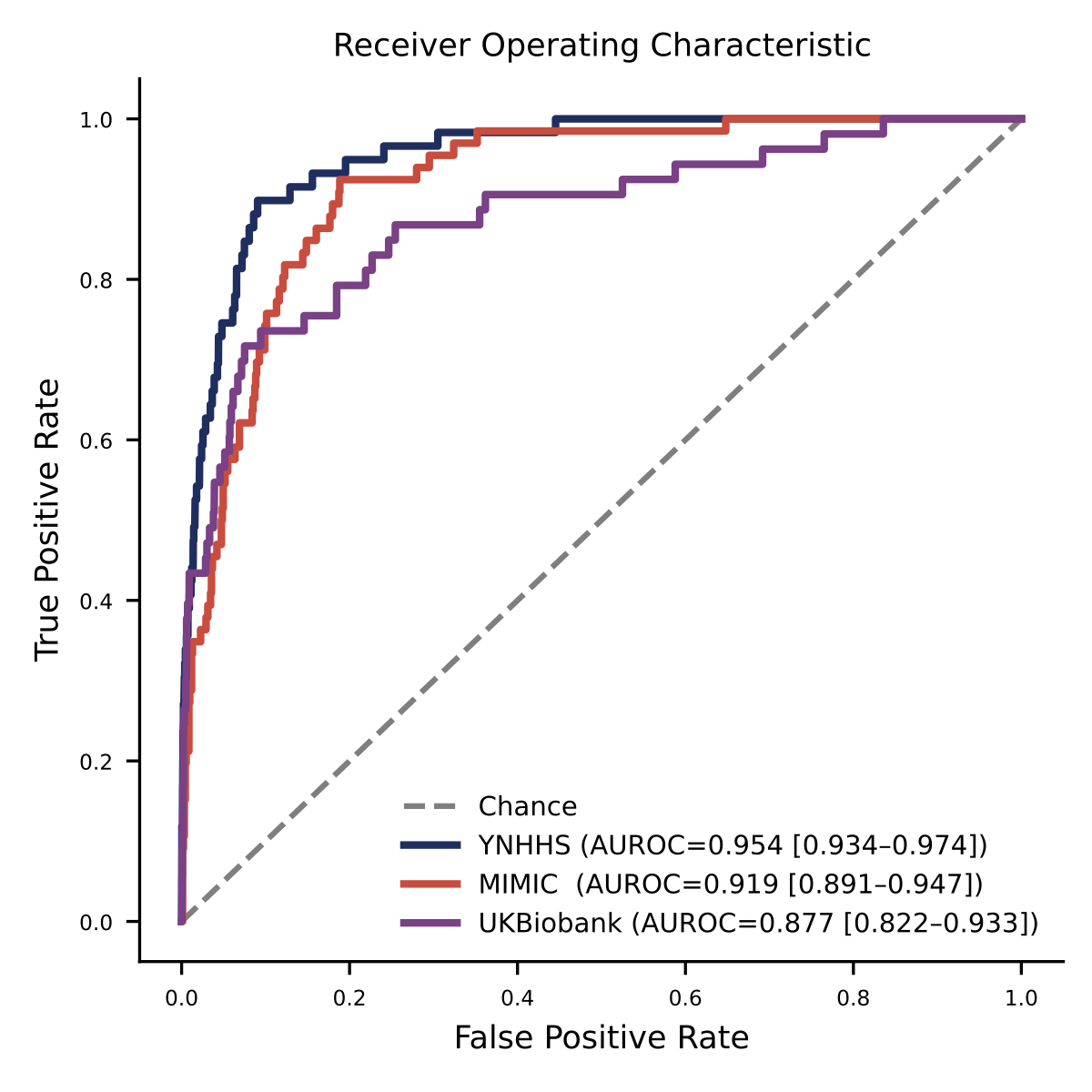
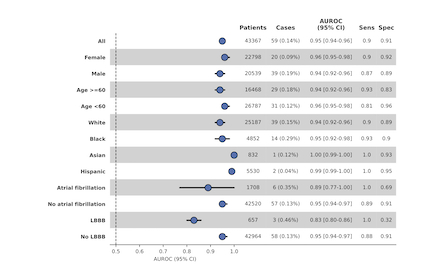
Results: The model demonstrated robust discrimination in internal validation with an area under the receiver operating curve (AUROC) of 0.95 (95% CI 0.93-0.97), sensitivity of 0.90 and specificity op 0.90 (Fig. 1). The performance remained consistent in external validation in MIMIC-IV (AUROC 0.92 [0.89-0.95]) and UK Biobank (AUROC 0.88 [0.82-0.93]). Stratified analyses demonstrated stable model performance across all demographic subgroups, including age (≥60,> 60 and <60), sex (men vs. women), and racial/ethnic subgroups. (Fig. 2) However, diagnostic accuracy was lower in patients with left bundle branch block or atrial fibrillation.
Conclusion: Our findings demonstrate that a noise-adapted AI-ECG model can effectively detect HCM from noisy, single-lead ECGs. This approach has the potential to enable scalable, automated, and accessible screening using wearable or portable ECG devices for the earlier identification of HCM in community settings.
Aim: We developed and validated a noise-adapted AI-ECG model specifically designed to detect HCM from noisy single-lead ECGs.
Methods: We developed an AI-ECG model using lead I from 160,396 unique 12-lead ECGs of 85,967 individuals in the Yale New Haven Health System (YNHHS) and augmented the ECG signal with real-world noises to develop noise-resilient models. A held-out test including 38,426 ECGs from unique individuals (mean age of 53.9 ± 19.3 years, 20,309 [53%] women) was used for internal validation. There were 59 (0.2%) HCM cases, adjudicated by expert clinicians using cardiac magnetic resonance (CMR) imaging. External validation was performed in manually validated MIMIC-IV (n=995, 66 HCM cases) and the UK Biobank (n=57,963, 53 HCM cases). To assess model fairness, we conducted stratified analyses by age, sex, race/ethnicity, and key ECG features.
Results: The model demonstrated robust discrimination in internal validation with an area under the receiver operating curve (AUROC) of 0.95 (95% CI 0.93-0.97), sensitivity of 0.90 and specificity op 0.90 (Fig. 1). The performance remained consistent in external validation in MIMIC-IV (AUROC 0.92 [0.89-0.95]) and UK Biobank (AUROC 0.88 [0.82-0.93]). Stratified analyses demonstrated stable model performance across all demographic subgroups, including age (≥60,> 60 and <60), sex (men vs. women), and racial/ethnic subgroups. (Fig. 2) However, diagnostic accuracy was lower in patients with left bundle branch block or atrial fibrillation.
Conclusion: Our findings demonstrate that a noise-adapted AI-ECG model can effectively detect HCM from noisy, single-lead ECGs. This approach has the potential to enable scalable, automated, and accessible screening using wearable or portable ECG devices for the earlier identification of HCM in community settings.
More abstracts on this topic:
A New Biomarker of Aging Derived From Electrocardiogram Improves Risk Prediction of Incident Myocardial Infarction and Stroke.
Wilsgaard Tom, Rosamond Wayne, Schirmer Henrik, Lindekleiv Haakon, Attia Zachi, Lopez-jimenez Francisco, Leon David, Iakunchykova Olena
A Cross-scale Causal Machine Learning Framework Pinpoints Mgl2+ Macrophage Orchestrators of Balanced Arterial GrowthHan Jonghyeuk, Kong Dasom, Schwarz Erica, Takaesu Felipe, Humphrey Jay, Park Hyun-ji, Davis Michael E