Final ID: MP2774
The inhibition of ATP citrate lyase prevents pathological cardiac fibrosis via the regulation of de novo lipogenesis and histone acetylation
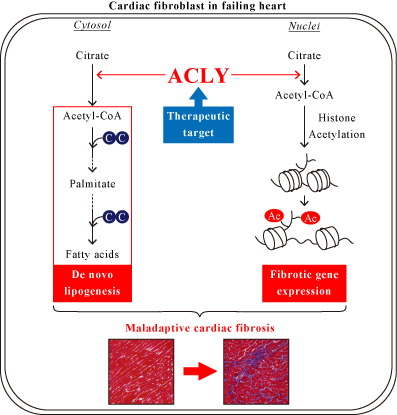
Abstract Body (Do not enter title and authors here): Background: Cardiac fibrosis is characterized by excessive extracellular matrix deposition in heart tissue that occurs early in heart failure (HF) and is caused not only by cardiac diseases such as ischemic heart disease and valvular diseases but also by lifestyle diseases such as hypertension and diabetes. Therefore, the inhibition of cardiac fibrosis has been considered an important therapeutic approach for treating HF. ATP citrate lyase (ACLY) is a key metabolic enzyme that converts mitochondria-derived citrate into acetyl-CoA. ACLY is essential for de novo lipogenesis (DNL) and histone acetylation, needs for cellular growth and organ fibrosis. Although fatty acids are required for energy production and biomass synthesis in the heart, the biological roles and regulatory mechanisms of ACLY-mediated DNL in pathological cardiac fibrosis remain largely unknown.
Hypothesis: The inhibition of ACLY reduces pathological cardiac fibrosis by suppress DNL and histone acetylation.
Methods: Adeno-associated virus serotype 9 (AAV9)-mediated shRNA targeting Acly was intravenously injected into C57BL/6J male mice from the retro-orbital sinus. The mice were subsequently continuously infused with a mixture of angiotensin II (AngII) and phenylephrine (PE) for two weeks. Cardiac phenotypes were evaluated via histological staining. Cell migration assays, stable isotope tracing with 13C-labeled glucose, and chromatin immunoprecipitation (ChIP) assays were performed using human cardiac fibroblasts (HCFs).
Results: ACLY expression was upregulated in the heart sections of mice treated with AngII/PE, especially in fibrotic areas. Masson’s trichrome staining and immunoblots revealed that gene silencing of Acly significantly reduced cardiac fibrosis and the expression of fibrous proteins in these mice. And gene silencing of Acly improves prognosis. SiRNA-mediated ACLY knockdown suppressed the proliferation and expression of fibrous proteins in cultured HCFs stimulated with transforming growth factor-β (TGF-β). Mechanistically, ACLY inhibition reduced DNL, limiting the fatty acid supply essential for cellular growth and proliferation; it also decreased H3K9 and H3K27 acetylation, in addition to the presence of acetylated H3K9 and H3K27 at the promoter regions of fibrotic genes.
Conclusion: Our findings demonstrated that ACLY plays pivotal roles in promoting pathological cardiac fibrosis. Targeting ACLY may emerge as a novel therapeutic strategy to prevent the progression of HF.
Hypothesis: The inhibition of ACLY reduces pathological cardiac fibrosis by suppress DNL and histone acetylation.
Methods: Adeno-associated virus serotype 9 (AAV9)-mediated shRNA targeting Acly was intravenously injected into C57BL/6J male mice from the retro-orbital sinus. The mice were subsequently continuously infused with a mixture of angiotensin II (AngII) and phenylephrine (PE) for two weeks. Cardiac phenotypes were evaluated via histological staining. Cell migration assays, stable isotope tracing with 13C-labeled glucose, and chromatin immunoprecipitation (ChIP) assays were performed using human cardiac fibroblasts (HCFs).
Results: ACLY expression was upregulated in the heart sections of mice treated with AngII/PE, especially in fibrotic areas. Masson’s trichrome staining and immunoblots revealed that gene silencing of Acly significantly reduced cardiac fibrosis and the expression of fibrous proteins in these mice. And gene silencing of Acly improves prognosis. SiRNA-mediated ACLY knockdown suppressed the proliferation and expression of fibrous proteins in cultured HCFs stimulated with transforming growth factor-β (TGF-β). Mechanistically, ACLY inhibition reduced DNL, limiting the fatty acid supply essential for cellular growth and proliferation; it also decreased H3K9 and H3K27 acetylation, in addition to the presence of acetylated H3K9 and H3K27 at the promoter regions of fibrotic genes.
Conclusion: Our findings demonstrated that ACLY plays pivotal roles in promoting pathological cardiac fibrosis. Targeting ACLY may emerge as a novel therapeutic strategy to prevent the progression of HF.
More abstracts on this topic:
5-oxoproline/ OPLAH Axis Alleviates Doxorubicin-induced Cardiomyopathy By Inhibiting Ferroptosis
Jiang Meng, Guo Xinning
Apelin Signaling Protects Against Experimental Pulmonary Hypertension-Induced Right Ventricular Remodeling Through Regulation of the Renin Angiotensin Aldosterone SystemBharti Manisha, Yakubov Bakhtiyor, Zagorski John, Albrecht Marjorie, Fisher Amanda, Cook Todd, Frump Andrea