Final ID: MP1728
MST1 Promotes the Development of Aortic Aneurysm and Dissection by Inhibiting FLNB-RAC1 Activity
Abstract Body (Do not enter title and authors here): Background: Aortic aneurysm and dissection (AAD) are life threatening cardiovascular emergencies while the pathogenesis mechanism remains unclear. Mammalian sterile 20-like kinase 1 (MST1) is a serine-threonine kinase involved in kinds of diseases while its role in AAD is unknown. This research aims to investigate the function of MST1 in the progression of AAD and explore effective pharmacotherapy strategy.
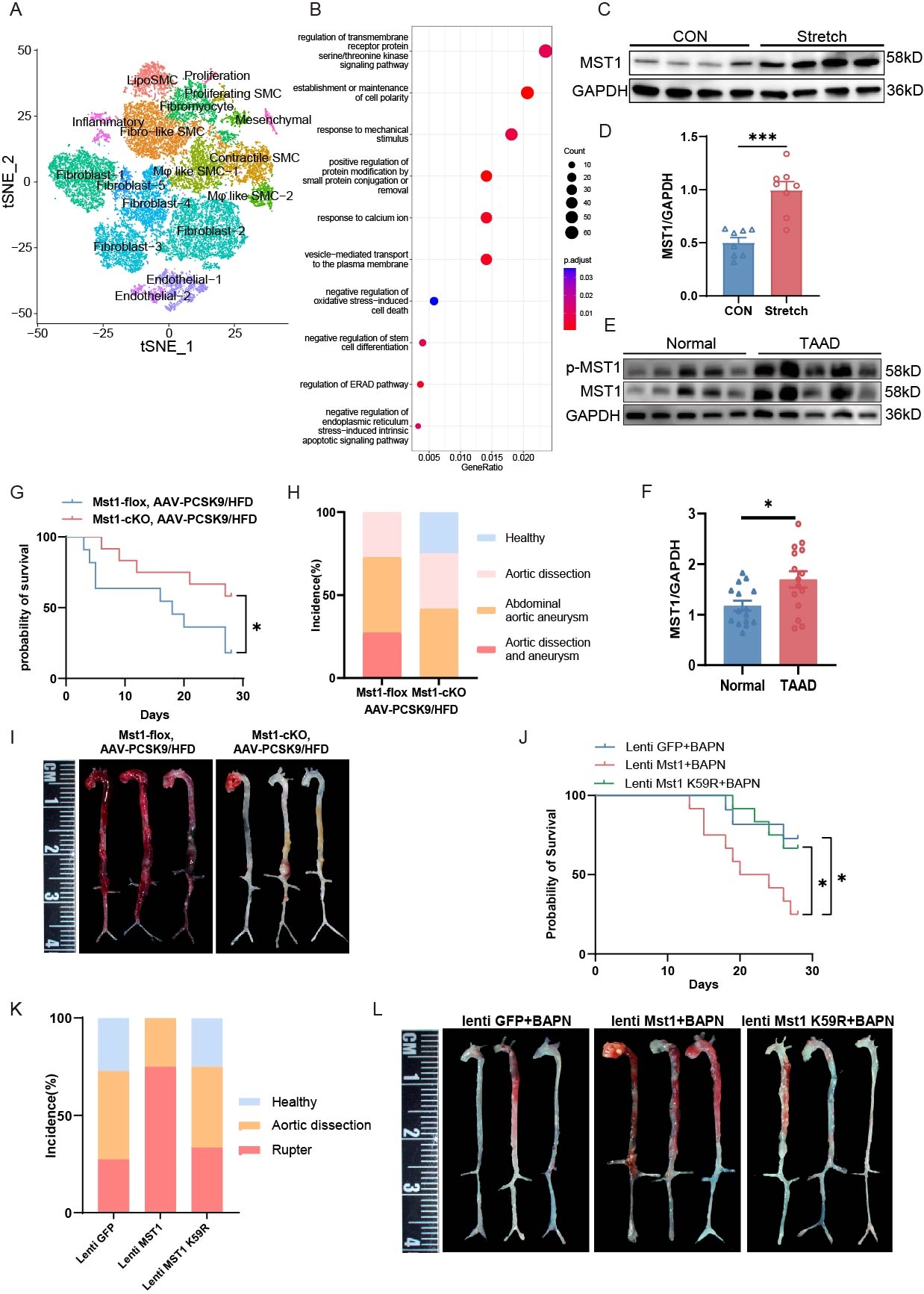
Methods: Single-cell RNA sequence (scRNA Seq) of human aortic dissection tissues was performed to reveal the key pathogenic molecules involved in disease progression. Mice with vascular smooth muscle cell (VSMC) specific Mst1 deletion or overexpression were generated, and the progression of AAD was determined. Immunoprecipitation-Mass spectrometry (IP-MS) and Phosphoproteomics were conducted to explore the downstream target and mechanism of MST1 in AAD. Molecular docking was performed to investigate the potential agonist of Rac1 and its effectiveness and safety were evaluated through animal model.
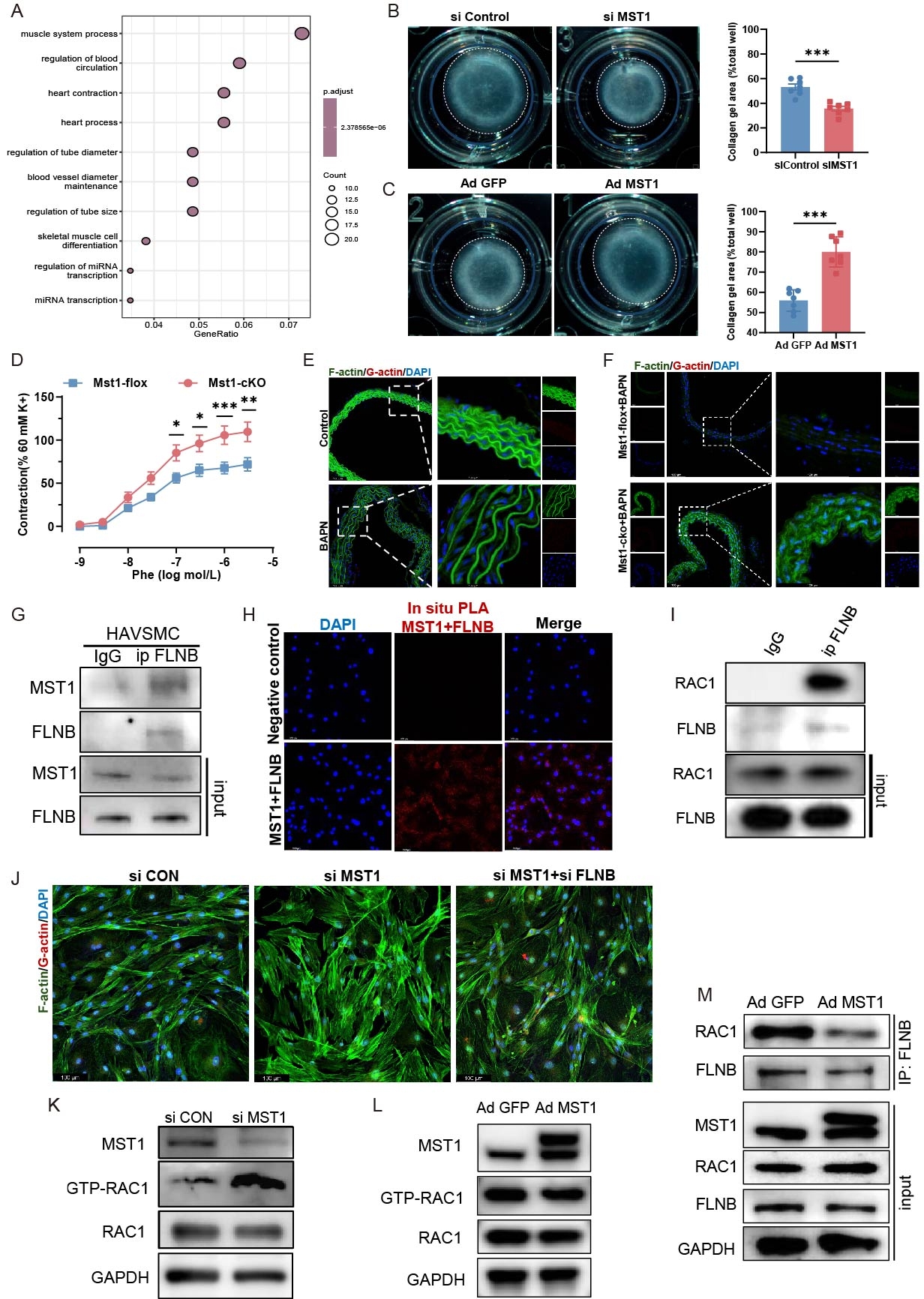
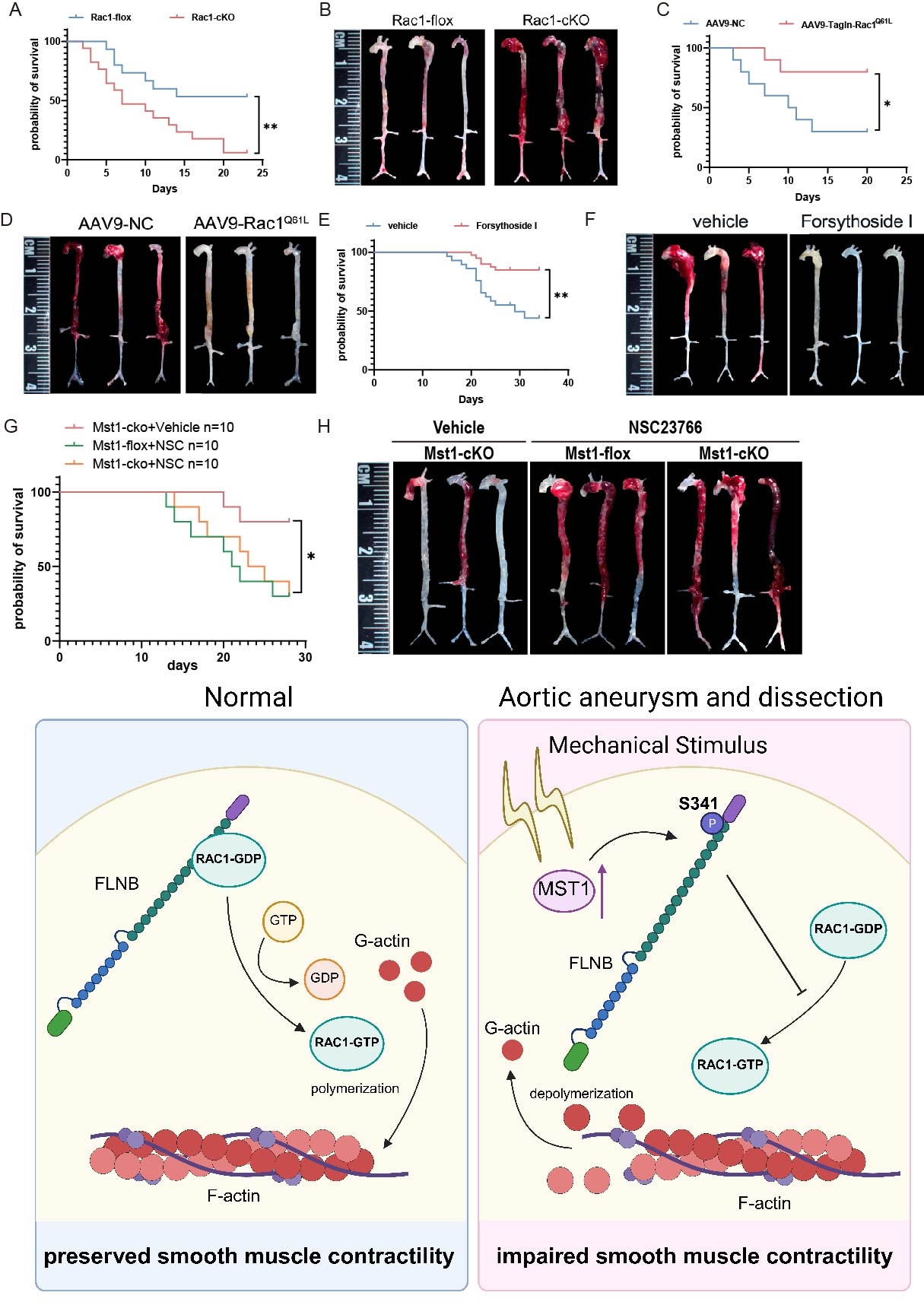
Results: scRNA Seq revealed serine-threonine kinase and response to mechanical stimulus signaling pathway changes in VSMC of human AAD tissues. MST1 expression was elevated in aortic tissues of patients with AAD. VSMC-specific Mst1 deficiency attenuated the progression of AAD, whereas lentivirus-mediated overexpression of wild-type Mst1, but not its kinase-deficient mutant, exacerbated disease severity. RNA-seq analysis indicated that MST1 deficiency enhanced vascular contractility. IP-MS combined with phosphoproteomic analysis identified FLNB and RAC1 as downstream targets of MST1. Further analysis showed that MST1 interacted with and phosphorylated FLNB at Ser341, which in turn disrupted FLNB-RAC1 interaction, suppressed RAC1 activity, and impaired F-actin VSMC contractility. VSMC-specific Rac1 deletion exacerbated, while VSMC-specific overexpression of Rac1Q61L gain of function mutation alleviated the progression of AAD. Forsythoside I was recognized to specifically bind to Q61 site and function as an agonist of Rac1 through Molecular docking. Animal model results showed that Forsythoside I had significant therapy effect for AAD with high safety.
Conclusions: Elevated MST1 expression inhibits FLNB-mediated RAC1 activation, leading to impaired vascular contractility, which serves as a key driver of AAD pathogenesis. Forsythoside I functions as an agonist of RAC1 significantly reduces AAD incidence, providing a potential therapeutic target for AAD treatment.
Methods: Single-cell RNA sequence (scRNA Seq) of human aortic dissection tissues was performed to reveal the key pathogenic molecules involved in disease progression. Mice with vascular smooth muscle cell (VSMC) specific Mst1 deletion or overexpression were generated, and the progression of AAD was determined. Immunoprecipitation-Mass spectrometry (IP-MS) and Phosphoproteomics were conducted to explore the downstream target and mechanism of MST1 in AAD. Molecular docking was performed to investigate the potential agonist of Rac1 and its effectiveness and safety were evaluated through animal model.
Results: scRNA Seq revealed serine-threonine kinase and response to mechanical stimulus signaling pathway changes in VSMC of human AAD tissues. MST1 expression was elevated in aortic tissues of patients with AAD. VSMC-specific Mst1 deficiency attenuated the progression of AAD, whereas lentivirus-mediated overexpression of wild-type Mst1, but not its kinase-deficient mutant, exacerbated disease severity. RNA-seq analysis indicated that MST1 deficiency enhanced vascular contractility. IP-MS combined with phosphoproteomic analysis identified FLNB and RAC1 as downstream targets of MST1. Further analysis showed that MST1 interacted with and phosphorylated FLNB at Ser341, which in turn disrupted FLNB-RAC1 interaction, suppressed RAC1 activity, and impaired F-actin VSMC contractility. VSMC-specific Rac1 deletion exacerbated, while VSMC-specific overexpression of Rac1Q61L gain of function mutation alleviated the progression of AAD. Forsythoside I was recognized to specifically bind to Q61 site and function as an agonist of Rac1 through Molecular docking. Animal model results showed that Forsythoside I had significant therapy effect for AAD with high safety.
Conclusions: Elevated MST1 expression inhibits FLNB-mediated RAC1 activation, leading to impaired vascular contractility, which serves as a key driver of AAD pathogenesis. Forsythoside I functions as an agonist of RAC1 significantly reduces AAD incidence, providing a potential therapeutic target for AAD treatment.
More abstracts on this topic:
Aortic Root Pseudoaneurysm Following Bicuspid Aortic Valve Endocarditis and Root Reconstruction
Odai Reuben, Kutilek Frank, Alchaer Anthony, Sajjad Laiba, Farhoud Hussam
A Rare Case of Mycotic Pseudoaneurysm in a Pediatric Patient with a History of Disseminated MRSAAmilhamja Anissa, Shaikh Adam, Porisch Mary