Final ID: MDP1663
Association between Pressure-Adjusted Heart Rate and Mortality in Cardiogenic Shock
Abstract Body (Do not enter title and authors here): Introduction: Among patients with cardiogenic shock (CS), high right atrial pressure (RAP) and low mean arterial pressure (MAP) are associated with in-hospital mortality. Pressure-adjusted heart rate (PAHR), defined as heart rate * RAP-to-MAP ratio (HR * [RAP/MAP]), integrates these parameters and has been used to identify cardiovascular dysfunction in critical illness. Higher PAHR values associate with higher surgical ICU mortality, but the prognostic significance of PAHR has not been assessed in cardiac intensive care unit (CICU) patients with CS.
Hypothesis: We hypothesized that higher PAHR values are associated with higher risk of in-hospital mortality in patients with CS.
Methods: The Critical Care Cardiology Trials Network (CCCTN) is a multinational registry of CICUs coordinated by the TIMI Study Group. Among CS admissions (2018-2023) undergoing invasive hemodynamic assessment within 24h of CICU admission, we calculated PAHR (in bpm) and assessed its relationship with in-hospital mortality. Patients on temporary mechanical circulatory support were excluded. Odds ratios were adjusted for age, sex, vasoactive-inotropic score (VIS), SCAI stage, and preceding cardiac arrest. Subgroup analyses stratifying by CS subtype were performed.
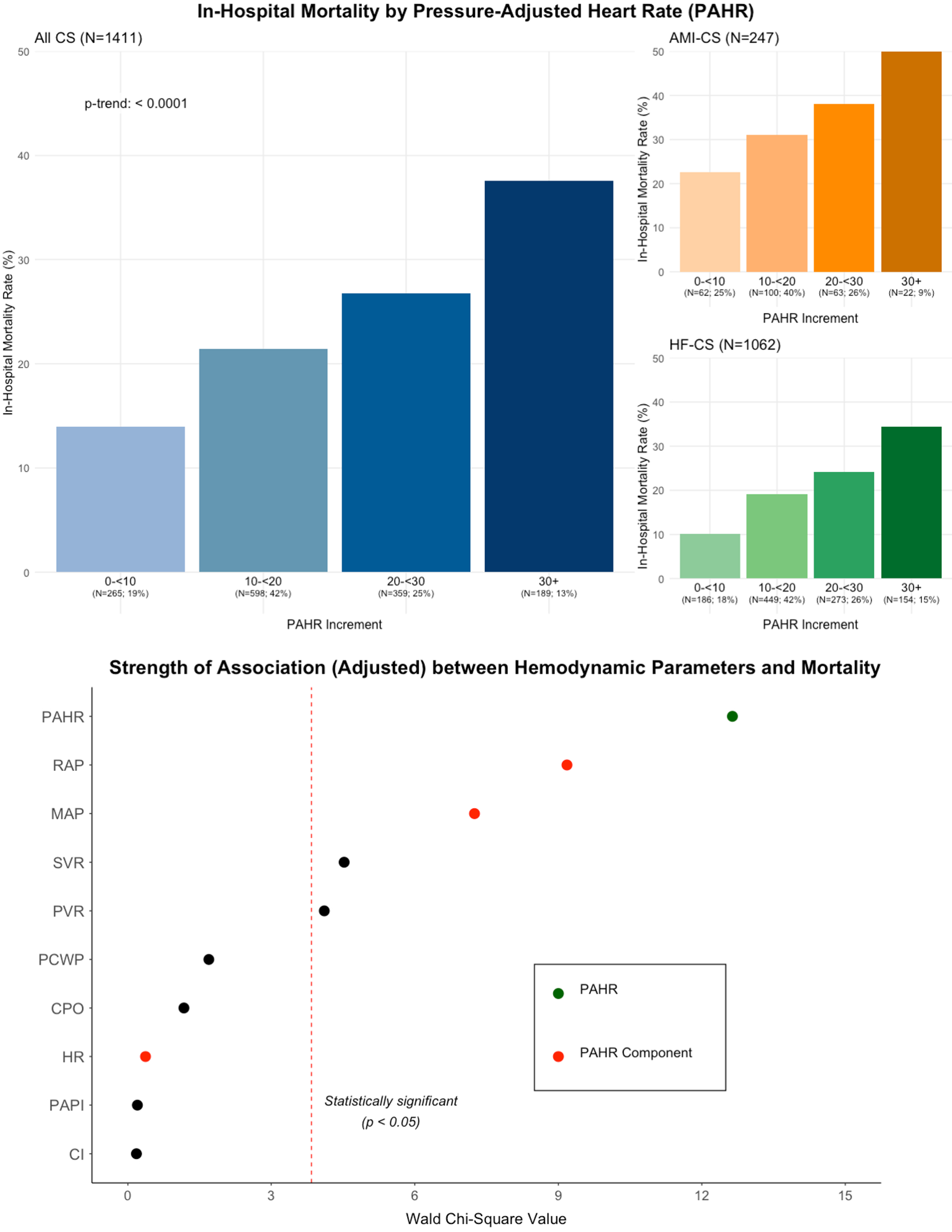
Results: Among the 1411 CS admissions in the analysis cohort (mean age 61 years, 31% women), 18% had AMI-CS, 75% had HF-CS, and 7% had secondary CS. At the time of assessment (median 1.4h after CICU admission), 75% were on vasoactive support (median VIS 3.8). Median HR was 92 (Q1-Q3 77-109) bpm, RAP 15 (Q1-Q3 10-19) mmHg, MAP 75 (Q1-Q3 67-87) mmHg, and PAHR 17 (Q1-Q3 11-24) bpm. A stepwise gradient of higher in-hospital mortality with increasing PAHR values (p-trend <0.001; Fig) was observed. In adjusted models, a higher PAHR was associated with higher in-hospital mortality (aOR per 10-bpm, 1.35 [95% CI, 1.15-1.58]), and had stronger prognostic associations with mortality than its individual hemodynamic components (Fig).
Conclusion: Higher PAHR is strongly associated with in-hospital mortality in CS patients. PAHR, a simple parameter which is readily calculable without needing a pulmonary artery catheter, may aid in identifying CS patients at higher risk of death.
Hypothesis: We hypothesized that higher PAHR values are associated with higher risk of in-hospital mortality in patients with CS.
Methods: The Critical Care Cardiology Trials Network (CCCTN) is a multinational registry of CICUs coordinated by the TIMI Study Group. Among CS admissions (2018-2023) undergoing invasive hemodynamic assessment within 24h of CICU admission, we calculated PAHR (in bpm) and assessed its relationship with in-hospital mortality. Patients on temporary mechanical circulatory support were excluded. Odds ratios were adjusted for age, sex, vasoactive-inotropic score (VIS), SCAI stage, and preceding cardiac arrest. Subgroup analyses stratifying by CS subtype were performed.
Results: Among the 1411 CS admissions in the analysis cohort (mean age 61 years, 31% women), 18% had AMI-CS, 75% had HF-CS, and 7% had secondary CS. At the time of assessment (median 1.4h after CICU admission), 75% were on vasoactive support (median VIS 3.8). Median HR was 92 (Q1-Q3 77-109) bpm, RAP 15 (Q1-Q3 10-19) mmHg, MAP 75 (Q1-Q3 67-87) mmHg, and PAHR 17 (Q1-Q3 11-24) bpm. A stepwise gradient of higher in-hospital mortality with increasing PAHR values (p-trend <0.001; Fig) was observed. In adjusted models, a higher PAHR was associated with higher in-hospital mortality (aOR per 10-bpm, 1.35 [95% CI, 1.15-1.58]), and had stronger prognostic associations with mortality than its individual hemodynamic components (Fig).
Conclusion: Higher PAHR is strongly associated with in-hospital mortality in CS patients. PAHR, a simple parameter which is readily calculable without needing a pulmonary artery catheter, may aid in identifying CS patients at higher risk of death.
More abstracts on this topic:
A Novel Echocardiography Risk Score Predicted Mortality In Patients With Heart Failure With Preserved Ejection Fraction.
Iwakura Katsuomi, Yoshio Yasumura, Hikoso Shungo, Okada Katsuki, Nakatani Daisaku, Sotomi Yohei, Sakata Yasushi, Tanaka Nobuaki, Okada Masato, Okamura Atsunori, Heitaro Watanabe, Seo Masahiro, Hayashi Takaharu, Yano Masamichi, Yamada Takahisa
A Comprehensive Study on Machine Learning Models Combining with Oversampling for One-year Persistent Coronary Artery Aneurysm in Kawasaki DiseaseLiang Kaizhi, Pang Yusheng, Su Danyan