Final ID: Th0029
Genetic Evidence for Glucose-dependent Insulinotropic Polypeptide as a Therapeutic Target for Peripheral Artery Disease
Abstract Body: Peripheral arterial disease (PAD) comorbid with type 2 diabetes (T2D) increases the risk of adverse limb events, with limited therapeutic options available. Recently liraglutide, a Glucagon-like Peptide-1 Receptor (GLP1R) agonist, has been shown to improve lower limb perfusion in PAD patients with T2D. Whether other incretin mimetics, such as gastric inhibitory peptide receptor (GIPR) agonists, can be used to treat PAD remains unclear. We hypothesized that genetic variation modeling incretin mimetics or cognate receptor activation protects against PAD.
We identified genetic associations with PAD using data from the Million Veteran Program (MVP) with mixed model logistic regression implemented in Regenie v2.0. Publicly available summary statistics for T2D from the DIAMANTE consortium (74,124 cases, 824,006 controls) and HbA1c (327,177 participants) were used for colocalization analyses and posterior probabilities (PP) of colocalization are reported. Logistic regression was used to test association of lead variants stratified by maximum Hba1c. Two-sample mendelian randomization was performed using lead variants at incretin loci, with T2D or Hba1c as the exposure and PAD as the outcome variable.
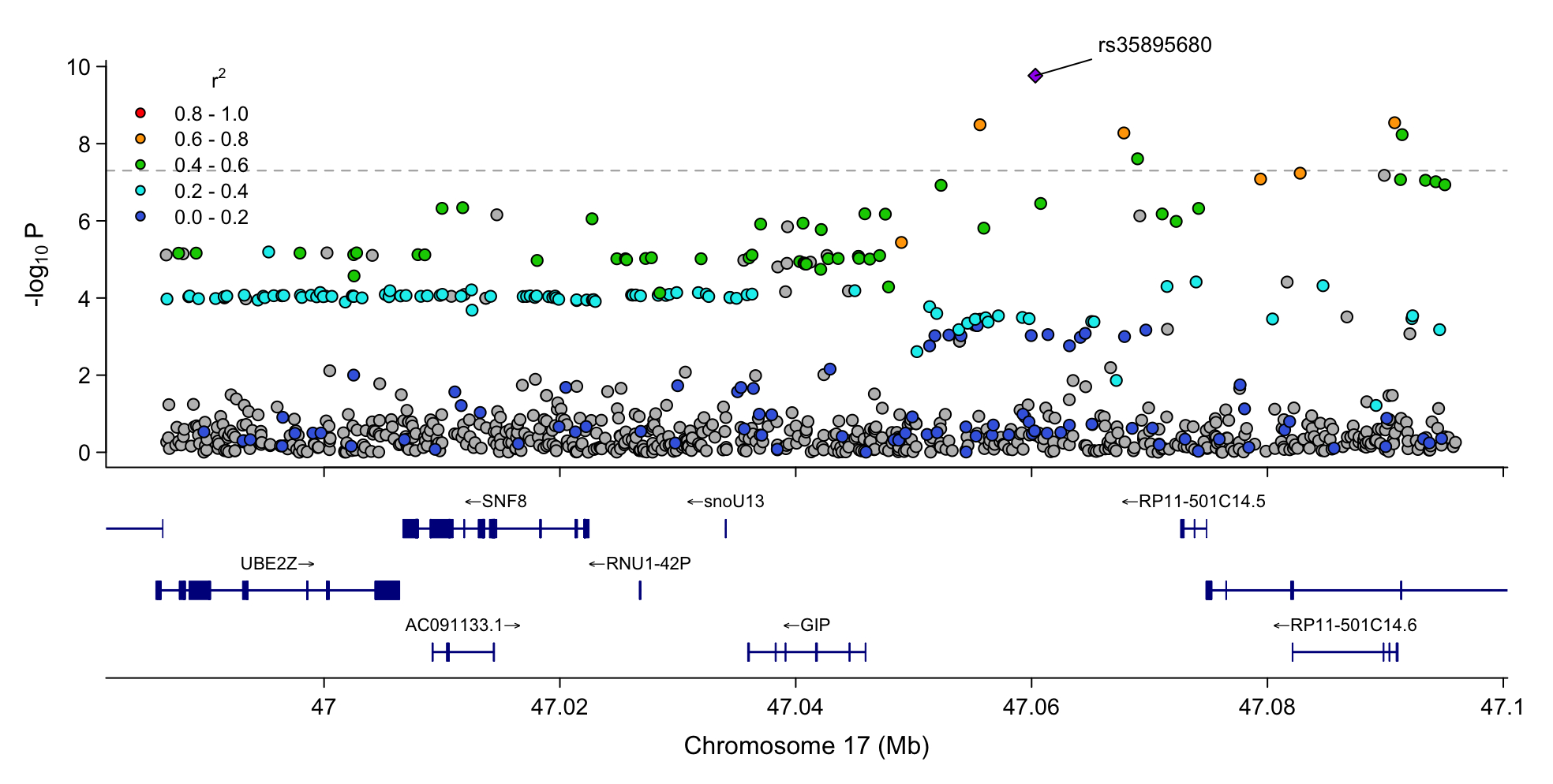
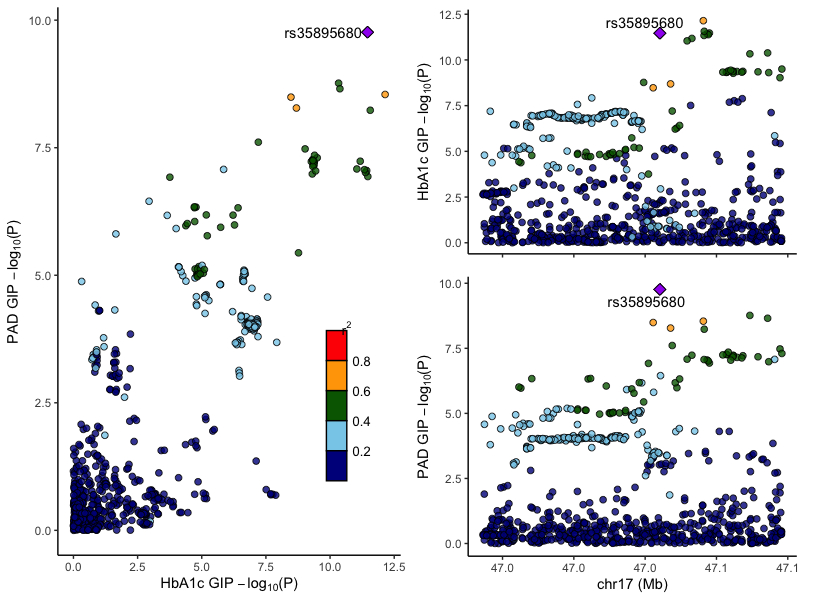
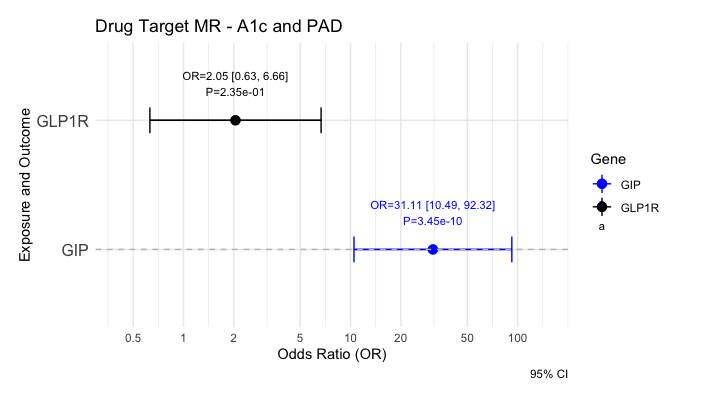
Our cohort consisted of 51,330 Veterans of European ancestry with PAD and 256,807 without PAD. We found genome-wide significant associations (P<5e-8) at the GIP locus with PAD (lead variant rs35895680; OR = 1.048, P = 1.723e-10), but no significant associations at GIPR, preproglucagon (GCG), or GLP1R (Fig 1). Genetic associations with PAD at this locus strongly colocalized with T2D and Hba1c signal (PAD-T2D, PP= 1.0; PAD-Hba1c, PP = 0.9) (Fig 2). Surprisingly, rs35895680 retained nominally significant associations with PAD across euglycemic (HbA1c < 5.7; OR = 1.0419, P = 1.94e-3), prediabetic (HbA1c 5.7–6.4; OR = 1.0509, P = 1.02e-3), and diabetic (HbA1c ≥ 6.5; OR = 1.0325, P = 9.88e-3) cohorts. Mendelian randomization supported a significant causal relationship between glycemic traits (T2D: OR = 2.69, P = 3.45e-10; HbA1c: OR = 31.11, P = 3.45e-10) and PAD through the lead variant at the GIP locus, but not through other incretin loci such as GLP1R (Fig 3).
This study suggests an association at the GIP locus with PAD, independent of diabetes. These findings may suggest the use of GIP mimetics may have an outsized benefit for PAD patients, with or without comorbid T2D. Functional experiments and clinical trials will be necessary to validate these findings.
We identified genetic associations with PAD using data from the Million Veteran Program (MVP) with mixed model logistic regression implemented in Regenie v2.0. Publicly available summary statistics for T2D from the DIAMANTE consortium (74,124 cases, 824,006 controls) and HbA1c (327,177 participants) were used for colocalization analyses and posterior probabilities (PP) of colocalization are reported. Logistic regression was used to test association of lead variants stratified by maximum Hba1c. Two-sample mendelian randomization was performed using lead variants at incretin loci, with T2D or Hba1c as the exposure and PAD as the outcome variable.
Our cohort consisted of 51,330 Veterans of European ancestry with PAD and 256,807 without PAD. We found genome-wide significant associations (P<5e-8) at the GIP locus with PAD (lead variant rs35895680; OR = 1.048, P = 1.723e-10), but no significant associations at GIPR, preproglucagon (GCG), or GLP1R (Fig 1). Genetic associations with PAD at this locus strongly colocalized with T2D and Hba1c signal (PAD-T2D, PP= 1.0; PAD-Hba1c, PP = 0.9) (Fig 2). Surprisingly, rs35895680 retained nominally significant associations with PAD across euglycemic (HbA1c < 5.7; OR = 1.0419, P = 1.94e-3), prediabetic (HbA1c 5.7–6.4; OR = 1.0509, P = 1.02e-3), and diabetic (HbA1c ≥ 6.5; OR = 1.0325, P = 9.88e-3) cohorts. Mendelian randomization supported a significant causal relationship between glycemic traits (T2D: OR = 2.69, P = 3.45e-10; HbA1c: OR = 31.11, P = 3.45e-10) and PAD through the lead variant at the GIP locus, but not through other incretin loci such as GLP1R (Fig 3).
This study suggests an association at the GIP locus with PAD, independent of diabetes. These findings may suggest the use of GIP mimetics may have an outsized benefit for PAD patients, with or without comorbid T2D. Functional experiments and clinical trials will be necessary to validate these findings.
More abstracts on this topic:
A Novel Machine Learning Strategy to Integrate Multi-Omics Data and Detect Genomic Loci and Gene-Environment Interactions for LDL Cholesterol
Li Changwei, Zhang Ruiyuan, Sun Yixi, Chen Jing, Wang Tao, Bazzano Lydia, Kelly Tanika, He Jiang
Analysis of Near-Infrared Spectroscopy Vascular Occlusion Test as a Complement to Ankle-Brachial Index and 6-Minute Walk Test in Patients Diagnosed with Peripheral Artery DiseaseRodriguez Cesar, Lanka Santh Prakash, Maraj Joshua, Alsabbagh Yaman, Farres Sam, Ade Carl, Liu Xiuwen, Delp Judy