Final ID: We0090
Genome wide association study of lower extremity aneurysms reveals shared genetic architecture with abdominal aortic aneurysms
Abstract Body: Introduction: Abdominal aortic aneurysms (AAA) are strongly correlated with lower extremity aneurysms (LEA), with AAA present in 50-90% of patients with femoral aneurysms and up to 50% in those with popliteal aneurysms. Conversely, around 14% of patients with AAA have concurrent popliteal aneurysms. While the genetic architecture of AAA has been well characterized, the genetic basis of lower extremity aneurysms (LEA) comprising those in the femoral and popliteal arteries remains unknown.
Methods:
We conducted a genome-wide association study for LEA in the Million Veterans Program. Veterans were divided into European, African and Hispanic cohorts based on genetically similarity to 1000 Genomes reference populations. Association testing was conducted for each respective population and results were combined with inverse variance weighted fixed effects meta-analysis. Genetic correlation between LEA, AAA, TAAD, and peripheral artery disease (PAD) were estimated using linkage disequilibrium score regression (LDSC). The top 5 lead variants were then examined for colocalization with AAA.
Results:
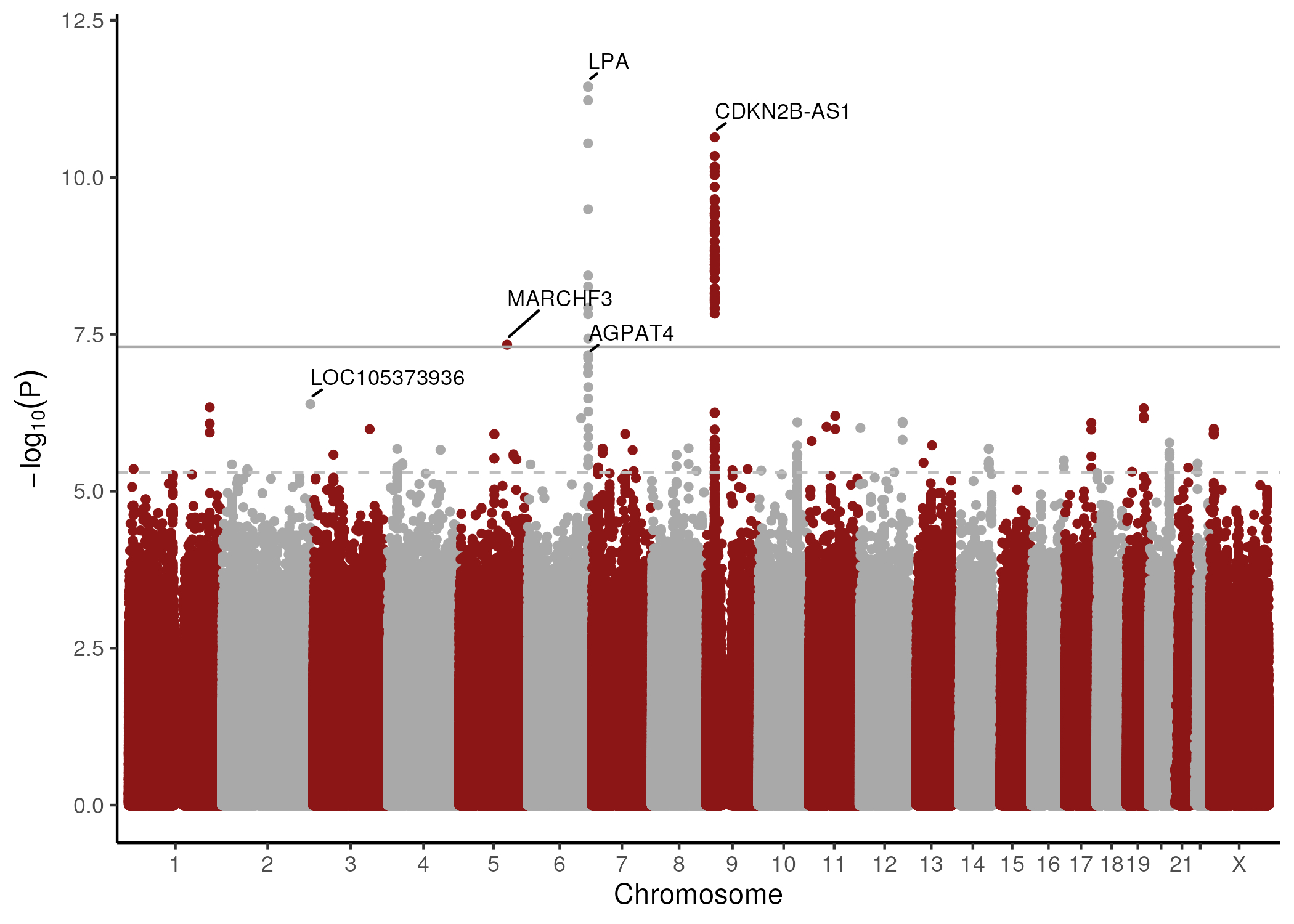
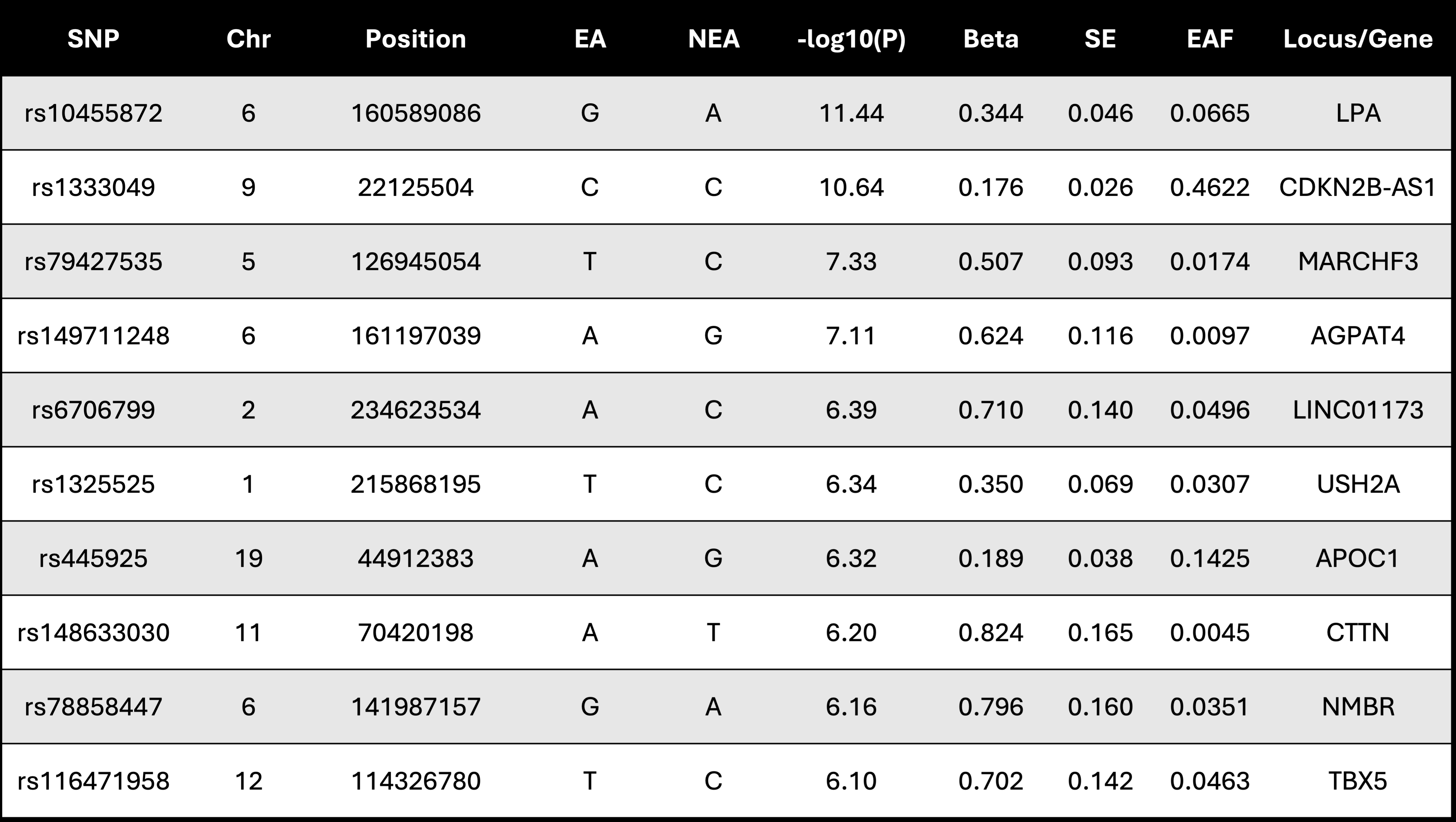
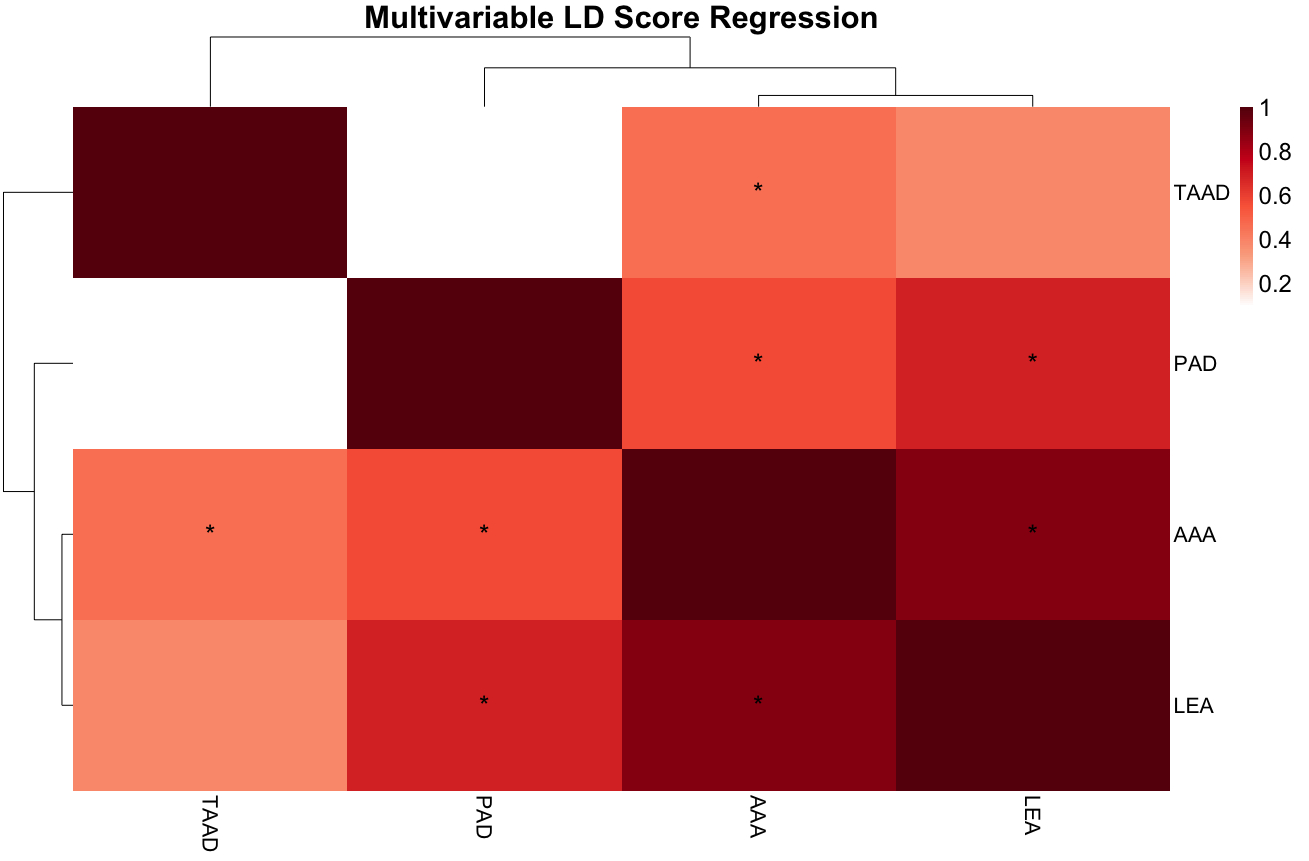
After quality control measures, approximately 25 million variants tested for association with LEA in 3,094 Veterans with LEA and 594,497 Veterans without LEA (Figure 1). We identified 63 genome-wide significant variants across three loci (p < 5 × 10-8) (Table 1). Additionally, 1,473 variants spanning 406 loci reached suggestive significance (p < 5 × 10-5). Genetic correlation analysis using LDSC demonstrated a strong shared genetic architecture between LEA and AAA (rg = 0.882, p = 1 × 10-7), a moderate correlation with PAD (rg = 0.687, p = 4 × 10-6), and weaker correlation with TAAD (rg = 0.391, p = 1 × 10-2) (Figure 2). Pairwise colocalization analysis revealed significant overlap between LEA and AAA at three of the top five lead variants, with a greater than 80% likelihood of the same causal genetic signal observed at the LPA, CDKN2B-AS1, and AGPAT4 loci.
Conclusion:
This study identifies 3 novel genome wide significant loci associated with LEA. Furthermore, we provide evidence for genetic contribution to the relationship between LEA and AAA – particularly at the LPA, CDKN2B-AS1 and AGAPT4 loci. These findings provide a foundation for further investigation into the genetic etiology of LEA.
Methods:
We conducted a genome-wide association study for LEA in the Million Veterans Program. Veterans were divided into European, African and Hispanic cohorts based on genetically similarity to 1000 Genomes reference populations. Association testing was conducted for each respective population and results were combined with inverse variance weighted fixed effects meta-analysis. Genetic correlation between LEA, AAA, TAAD, and peripheral artery disease (PAD) were estimated using linkage disequilibrium score regression (LDSC). The top 5 lead variants were then examined for colocalization with AAA.
Results:
After quality control measures, approximately 25 million variants tested for association with LEA in 3,094 Veterans with LEA and 594,497 Veterans without LEA (Figure 1). We identified 63 genome-wide significant variants across three loci (p < 5 × 10-8) (Table 1). Additionally, 1,473 variants spanning 406 loci reached suggestive significance (p < 5 × 10-5). Genetic correlation analysis using LDSC demonstrated a strong shared genetic architecture between LEA and AAA (rg = 0.882, p = 1 × 10-7), a moderate correlation with PAD (rg = 0.687, p = 4 × 10-6), and weaker correlation with TAAD (rg = 0.391, p = 1 × 10-2) (Figure 2). Pairwise colocalization analysis revealed significant overlap between LEA and AAA at three of the top five lead variants, with a greater than 80% likelihood of the same causal genetic signal observed at the LPA, CDKN2B-AS1, and AGPAT4 loci.
Conclusion:
This study identifies 3 novel genome wide significant loci associated with LEA. Furthermore, we provide evidence for genetic contribution to the relationship between LEA and AAA – particularly at the LPA, CDKN2B-AS1 and AGAPT4 loci. These findings provide a foundation for further investigation into the genetic etiology of LEA.
More abstracts on this topic:
Adipose tissue extracellular vesicles mediate pro-arrhythmic changes in atrial cardiomyocytes
Limpitikul Worawan, Garcia Contreras Marta, Betti Michael, Sheng Quanhu, Xiao Ling, Chatterjee Emeli, Gamazon Eric, Shah Ravi, Das Saumya
A drug target Mendelian randomization study of triglyceride lowering therapies for aortic stenosisCiofani Jonathan, Han Daniel, Gill Dipender, Rao Karan, Allahwala Usaid, Bhindi Ravinay