Final ID: MP1074
Adipose tissue extracellular vesicles mediate pro-arrhythmic changes in atrial cardiomyocytes
Abstract Body (Do not enter title and authors here): Background: Obesity increases the risk of arrhythmias, such as atrial fibrillation. How increased adiposity alters the properties of cardiac tissue is largely unknown. Extracellular vesicles (EVs), membrane-bound particles containing nucleic acid, proteins, and metabolites, released from adipose tissue, may mediate communication with the heart, contributing to electrical or structural remodeling.
Method: First, to study adipose-to-heart signaling, transgenic mice expressing Neon-Green-tagged EVs originating from adipose tissue (adipo-EVs) were fed with either a regular or high-fat diet for 16 weeks, and the target tissue was analyzed using Western blot. Second, to examine the effects of adipo-EVs on the electrical properties of cardiac tissue, induced pluripotent stem cell-derived atrial cardiomyocytes (iPSC-aCMs) were treated with EVs, derived from visceral fat biopsies of bariatric surgery patients, prior to functional characterization. To dissect the mechanism underlying the observed electrical changes, bulk RNA sequencing was performed on treated cardiomyocytes to identify differentially expressed genes (DEGs), which were later analyzed using multi-ancestry GWAS. Lastly, candidate DEG targets were pharmacologically tested in vitro.
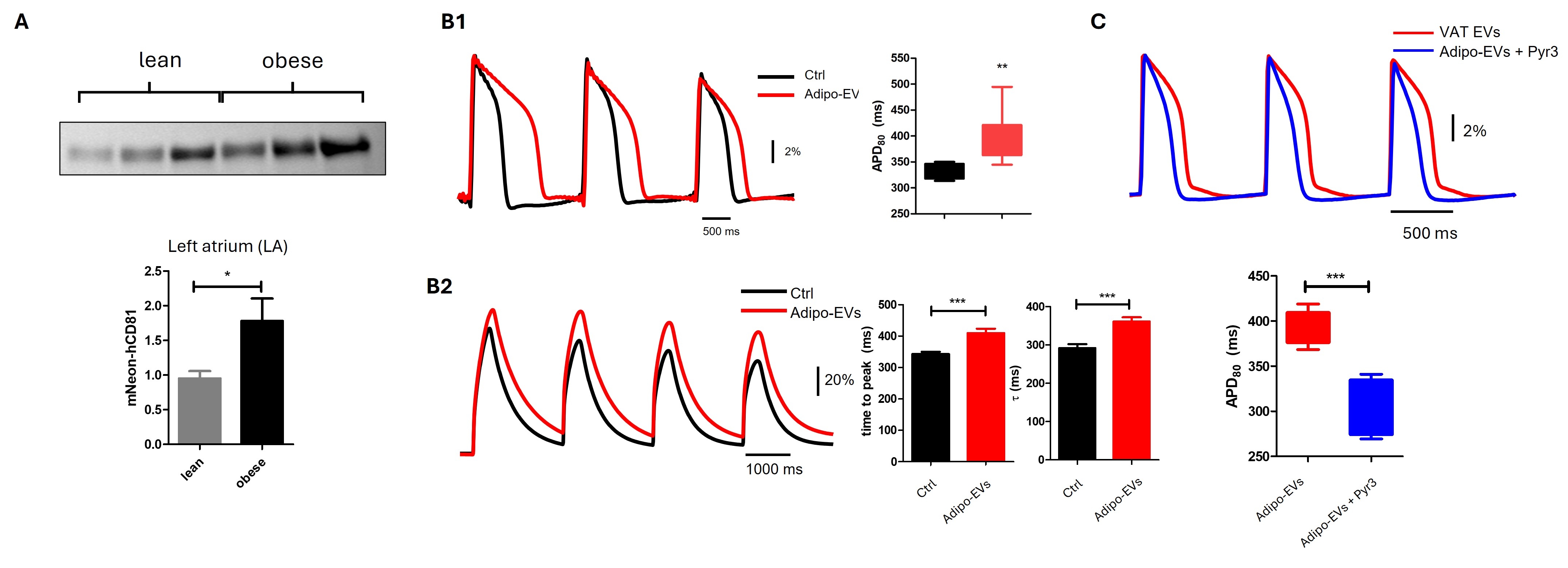
Results: (1) Atria of obese mice showed significantly more Neon-Green adipo-EVs than lean controls, indicating enhanced adipose-to-heart EV trafficking (panel A, n=3/group, p<0.05). (2) IPSC-aCMs treated with human adipo-EVs showed a significant prolongation of action potential duration (APD), increased incidence of arrhythmias (panel B1, n=5 patients, p<0.01), and impaired calcium dynamics (panel B2, n=3 patients, p<0.05). RNA sequencing of adipo-EV-treated iPSC-aCMs demonstrated DEGs linked to pathways and targets associated with atrial fibrillation and QT prolongation in GWAS studies. (3) Experimental testing of identified DEGs prioritized TRPC3 as an important mediator of the adipo-EV effects: inhibition of TRPC3 by its inhibitor Pyr3 restored APD in adipo-EV-treated atrial myocytes (panel C, n=3 patients, p<0.001).
Conclusion: EV-mediated communication between adipose and cardiac tissue may contribute to pro-arrhythmic properties of cardiac tissue in obesity, including prolongation of APD and dysregulation of calcium transient. Further investigation of the EV cargo and its potential interaction with DEGs could identify additional druggable targets and shed light on the mechanism underlying obesity-related arrhythmias.
Method: First, to study adipose-to-heart signaling, transgenic mice expressing Neon-Green-tagged EVs originating from adipose tissue (adipo-EVs) were fed with either a regular or high-fat diet for 16 weeks, and the target tissue was analyzed using Western blot. Second, to examine the effects of adipo-EVs on the electrical properties of cardiac tissue, induced pluripotent stem cell-derived atrial cardiomyocytes (iPSC-aCMs) were treated with EVs, derived from visceral fat biopsies of bariatric surgery patients, prior to functional characterization. To dissect the mechanism underlying the observed electrical changes, bulk RNA sequencing was performed on treated cardiomyocytes to identify differentially expressed genes (DEGs), which were later analyzed using multi-ancestry GWAS. Lastly, candidate DEG targets were pharmacologically tested in vitro.
Results: (1) Atria of obese mice showed significantly more Neon-Green adipo-EVs than lean controls, indicating enhanced adipose-to-heart EV trafficking (panel A, n=3/group, p<0.05). (2) IPSC-aCMs treated with human adipo-EVs showed a significant prolongation of action potential duration (APD), increased incidence of arrhythmias (panel B1, n=5 patients, p<0.01), and impaired calcium dynamics (panel B2, n=3 patients, p<0.05). RNA sequencing of adipo-EV-treated iPSC-aCMs demonstrated DEGs linked to pathways and targets associated with atrial fibrillation and QT prolongation in GWAS studies. (3) Experimental testing of identified DEGs prioritized TRPC3 as an important mediator of the adipo-EV effects: inhibition of TRPC3 by its inhibitor Pyr3 restored APD in adipo-EV-treated atrial myocytes (panel C, n=3 patients, p<0.001).
Conclusion: EV-mediated communication between adipose and cardiac tissue may contribute to pro-arrhythmic properties of cardiac tissue in obesity, including prolongation of APD and dysregulation of calcium transient. Further investigation of the EV cargo and its potential interaction with DEGs could identify additional druggable targets and shed light on the mechanism underlying obesity-related arrhythmias.
More abstracts on this topic:
A drug target Mendelian randomization study of triglyceride lowering therapies for aortic stenosis
Ciofani Jonathan, Han Daniel, Gill Dipender, Rao Karan, Allahwala Usaid, Bhindi Ravinay
Calbindin 2 as A Novel Biomarker and Therapeutic Target for Abdominal Aortic Aneurysm: Integrative Analysis of Human Proteomes and GeneticsBao Yulin, Zhou Liu-hua, Wang Liansheng