Final ID:
Long-term Efficacy and Safety of Lerodalcibep in the Open-label 72-week Extension Study of Subjects Previously on Inclisiran or Lerodalcibep in the LIBerate-VI Trial
Abstract Body (Do not enter title and authors here): Background: Lerodalcibep, a recombinant fusion protein PCSK9-inhibitor, and inclisiran, a siRNA inhibiting hepatic PCSK9 production both provided effective LDL-cholesterol (LDL-C) reduction in a phase 3 trial with subjects randomized to either lerodalcibep or inclisiran. On completion subjects were eligible to enter an open-label extension (OLE) trial on lerodalcibep.
Methods: Subjects participating in the OLE study received lerodalcibep 300 mg SC monthly for 72 weeks. They were dosed and monitored in clinic for 12 weeks after which they were seen in clinic 3 monthly. Primary and secondary endpoints were LDL-C changes at Week 72 and other lipids, achievement of LDL-C guidelines and safety respectively.
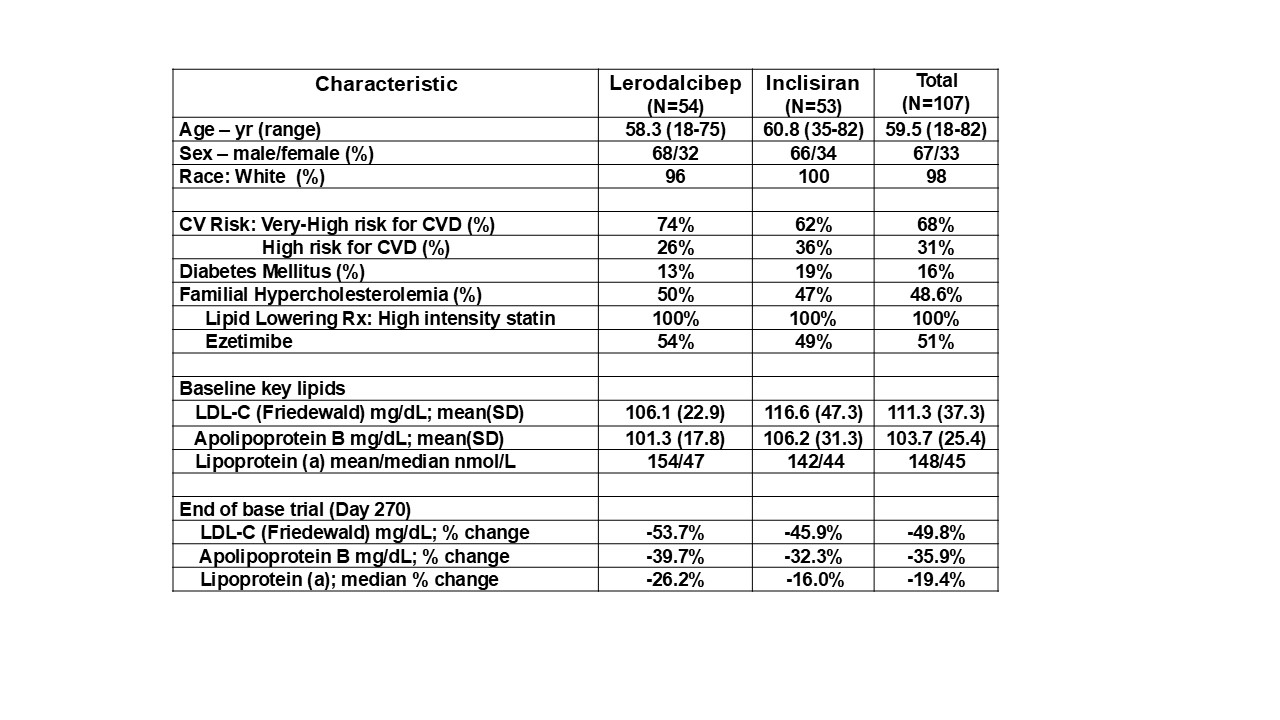
Results: Of 117 (73%) entering the OLE 107 (91%) completed all 72 weeks. Table 1 shows baseline data at entry and completion of base trial. At the 72-week primary endpoint, mean and absolute LDL-C reductions were 59.2% and 66.6 mg/dL respectively, with time average reductions across all visits (Weeks 4,8,12,24,36, 48, 60 and 72 ) of 63.4% and 71.4 mg/dL respectively. Mean ApoB was reduced by 45.3% and median Lp(a) by 35.5%. LDL-C response over 72 weeks was consistent in those previously on lerodalcibep. Subjects switched from inclisiran had substantial additional reductions 30% to 35% through week 24 which remained 22% lower through week 72. Similar additional reductions were seen in Apo B and Lp(a) and subjects achieving guideline goals rose significantly. Lerodalcibep was well tolerated, with no treatment related serious adverse events.
Conclusions: Treatment with lerodalcibep demonstrated consistent long-term efficacy in those previously treated with the drug. Subjects switched from inclisiran to lerodalcibep had prolonged substantial additional LDL-C reductions and goal achievement, consistent with added inhibition of circulating free PCSK from non-hepatic synthesis.
Methods: Subjects participating in the OLE study received lerodalcibep 300 mg SC monthly for 72 weeks. They were dosed and monitored in clinic for 12 weeks after which they were seen in clinic 3 monthly. Primary and secondary endpoints were LDL-C changes at Week 72 and other lipids, achievement of LDL-C guidelines and safety respectively.
Results: Of 117 (73%) entering the OLE 107 (91%) completed all 72 weeks. Table 1 shows baseline data at entry and completion of base trial. At the 72-week primary endpoint, mean and absolute LDL-C reductions were 59.2% and 66.6 mg/dL respectively, with time average reductions across all visits (Weeks 4,8,12,24,36, 48, 60 and 72 ) of 63.4% and 71.4 mg/dL respectively. Mean ApoB was reduced by 45.3% and median Lp(a) by 35.5%. LDL-C response over 72 weeks was consistent in those previously on lerodalcibep. Subjects switched from inclisiran had substantial additional reductions 30% to 35% through week 24 which remained 22% lower through week 72. Similar additional reductions were seen in Apo B and Lp(a) and subjects achieving guideline goals rose significantly. Lerodalcibep was well tolerated, with no treatment related serious adverse events.
Conclusions: Treatment with lerodalcibep demonstrated consistent long-term efficacy in those previously treated with the drug. Subjects switched from inclisiran to lerodalcibep had prolonged substantial additional LDL-C reductions and goal achievement, consistent with added inhibition of circulating free PCSK from non-hepatic synthesis.
More abstracts on this topic:
Advanced Lipid Status Parameters in Women With Preeclampsia
Gojkovic Tamara, Saric Matutinovic Marija, Ivanisevic Jasmina, Vladimirov Sandra, Spasojevic Kalimanovska Vesna, Mikovic Zeljko, Stefanovic Aleksandra, Ardalic Daniela, Antonic Tamara, Banjac Gorica, Zeljkovic Aleksandra, Vekic Jelena, Miljkovic Trailovic Milica, Munjas Jelena, Jovicic Snezana
A Novel Approach to Manage Hypercholesterolemia: The Veterans Affairs Lipid Optimization Reimagined Quality Improvement (VALOR-QI) ProgramDjousse Luc, Leesch Tharen, Pena David, Gaziano Michael, Ward Rachel, Wellman Helen, Yel Nedim, Santos Abigail, Delgrande Jen, Fink Abigail, Colson Kristin, Pan Eddie