Final ID: 4170118
Efficacy and Safety of Lerodalcibep, a Third Generation PCSK9 Inhibitor, in 703 Heterozygous Familial Hypercholesterolemia Subjects in the Open Label Extension Trial
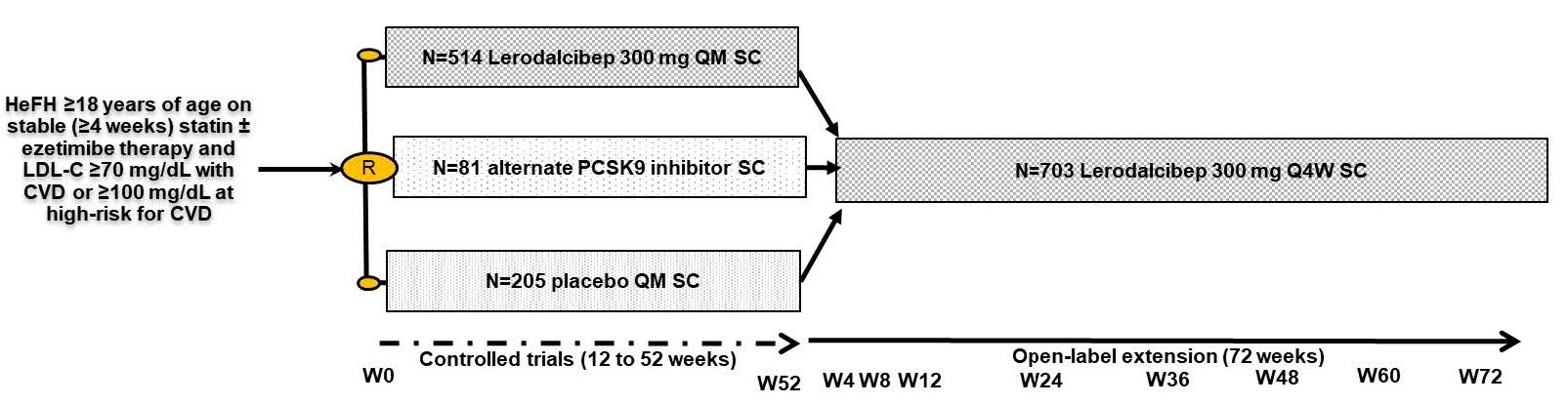
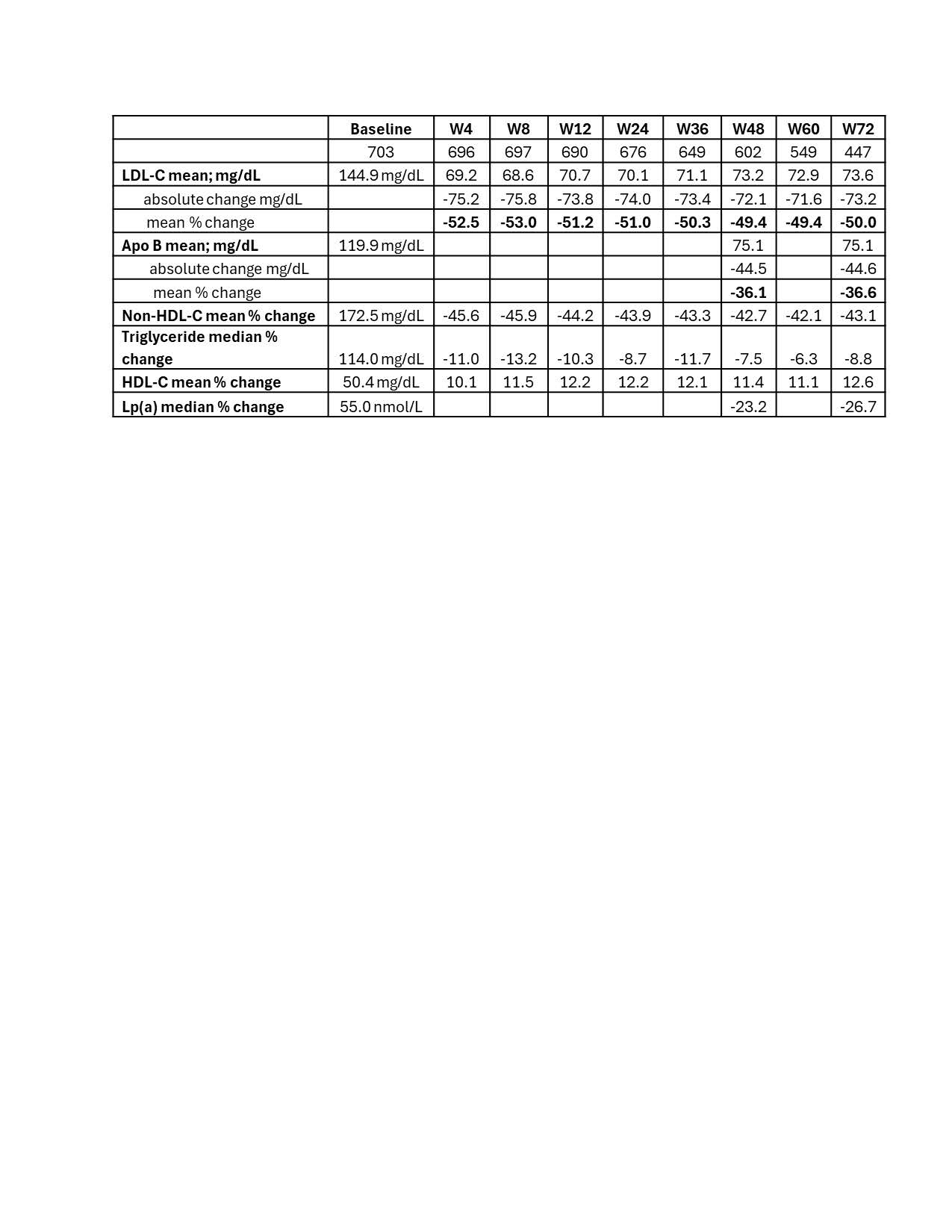
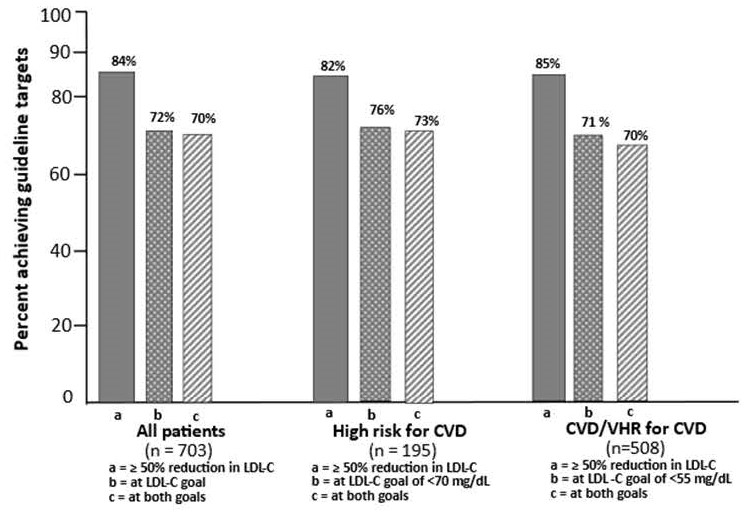
Abstract Body (Do not enter title and authors here): Background: We report results from 800 heterozygous familial hypercholesterolemia (HeFH) patients randomized to 5 phase 3 studies with lerodalcibep, a third generation PCSK9 inhibitor, with 703 (88%) completing the final visit of each trial and continuing in an ongoing 72 week open-label extension (OLE) study. All patients received lerodalcibep 300 mg monthly in the OLE, with over 60% having received lerodalcibep for 2 years including the parent trial. The primary objectives of the OLE were to assess the long-term safety, tolerability, and efficacy after 48 and 72 weeks. Methods: Of the 703 HeFH subjects, 448 (64%) received lerodalcibep for between 12 and 52 weeks, and 255 (36%) placebo or other PCSK9 directed therapies in the base trials – Figure 1. In the OLE, all patients received lerodalcibep immediately on completing the final base study visit, or within 28 days, and then monthly. The primary efficacy endpoints were LDL-C reduction achieved after 48 and 72 weeks of the OLE study. Secondary endpoints included the achievement of currently recommended LDL-C targets for high or very-high risk for ASCVD, safety, and changes in other lipid parameters during the OLE. Results: Mean age (range) was 53.8 (18-80) years, 47% female, 88% white and 12% South Asian, multiracial or black with 72% at very high risk and 28% at high risk for ASCVD. Despite treatment with statin in 91% (high intensity 71%) and ezetimibe in 52%, the mean (SD) LDL-C at baseline was 144.9 (61.9) mg/dL and mean apolipoprotein B 119.9 (39.3) mg/dL. Mean and absolute reductions in LDL-C at visits were consistent, ranging between 49.4% and 53% and 71.6 and 75.6 mg/dL through 72 weeks -Table 1. Mean (SD) apolipoprotein B and median (IQR) lipoprotein(a), measured only at weeks 48 and 72, were reduced by 36.1 (24.4)% to 36.6 (23.5)% and by 23.2 (-5.9;-41.3)% to 26.7 (-6.6;-42.9)% respectively. During the OLE≥70% of subjects achieved both a reduction in LDL-C ≥50% and LDL-C target irrespective of ASCVD risk on lerodalcibep - Figure 2. Injection site adverse events, predominantly mild and transient, were the only adverse events considered related to lerodalcibep. Conclusions: Lerodalcibep 300 mg QM administered for up to 2 years in a large cohort of over 700 HeFH patients, significantly, persistently and consistently reduced LDL-C and other atherogenic lipids with no attenuation and good tolerability and safety. Over 70% of HeFH subjects were able to achieve currently recommended LDL-C targets.
More abstracts on this topic:
A Case of Recurrent Acute Coronary Syndrome and Cardiogenic Shock due to Apolipoprotein A-IV Amyloidosis
Muthukkumar Rashmi, Holmes Taylor, Friede Kevin
Effect of Evolocumab in Patients at High Cardiovascular Risk without Prior Myocardial Infarction or Stroke: Primary Results of the VESALIUS-CV trialBohula Erin, Wang Huei, Cyrille Marcoli, Paiva Da Silva Lima Gabriel, Giugliano Robert, Sabatine Marc, Marston Nicholas, Bhatia Ajay, De Ferrari Gaetano Maria, Leiter Lawrence, Nicolau Jose, Park Jeong-gun, Murphy Sabina, Walsh Emileigh