Final ID: 4165221
Long-term Efficacy of Lerodalcibep in 1,468 Patients at Very High and High Risk for CVD in the 72 week Open-Label Extension Trial
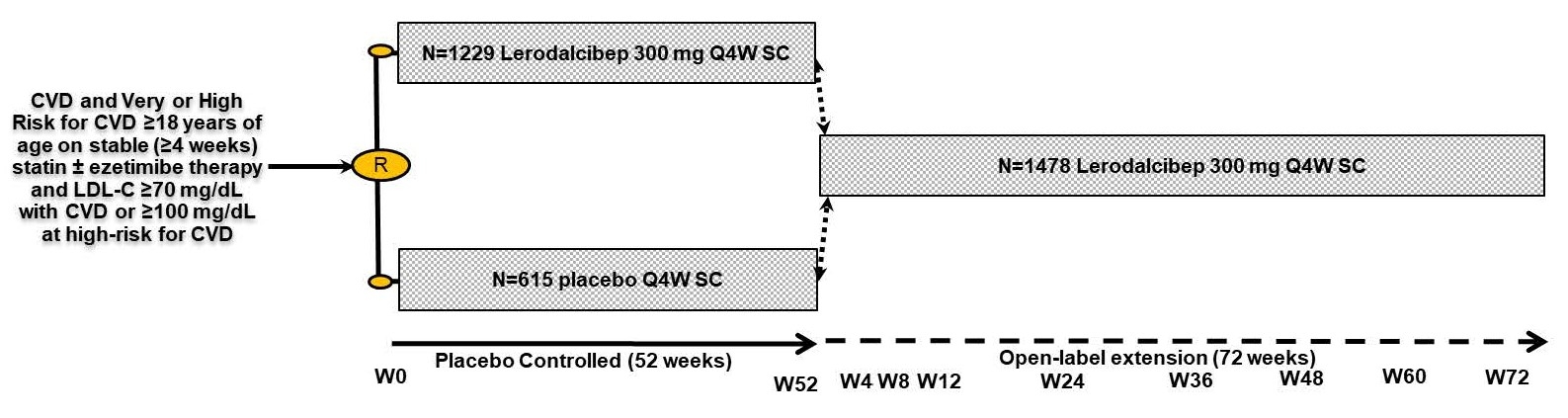
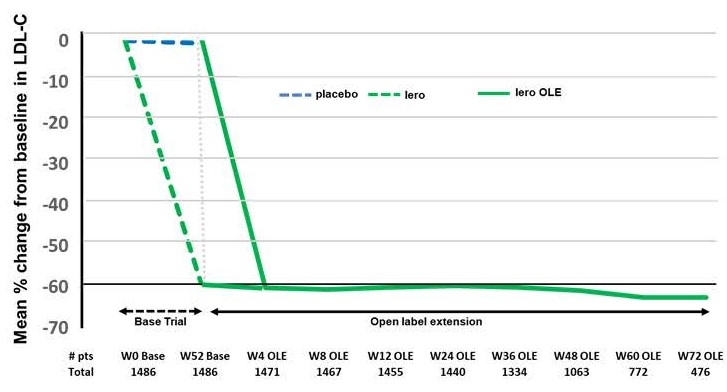
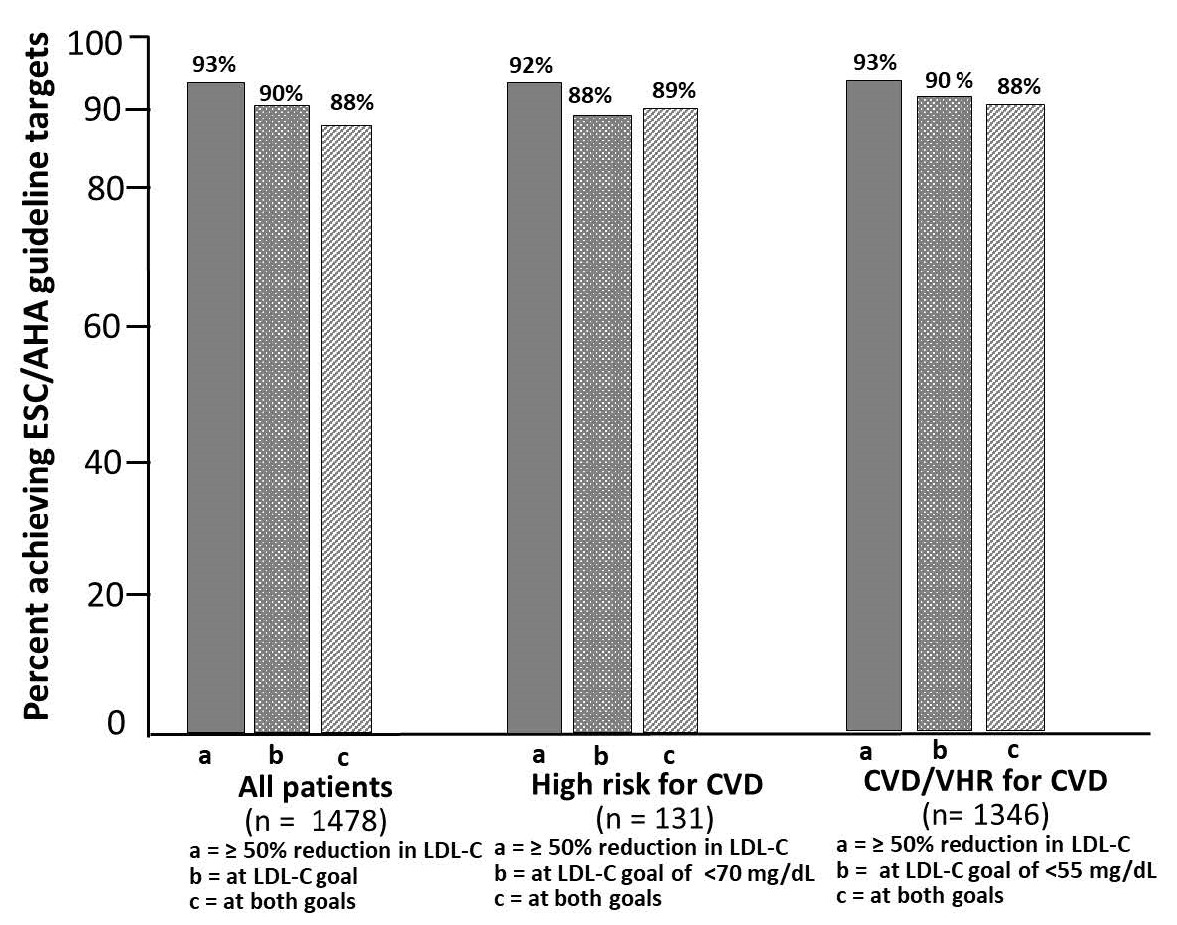
Abstract Body (Do not enter title and authors here): Background: Lerodalcibep, a small binding protein (adnectin) PCSK9 inhibitor reduced placebo-adjusted LDL-cholesterol (LDL-C) by a mean (SE) of 59.1(1.47) % with monthly 300 mg SC 1.2 mL dosing in the 1844 patient 52-week Phase III LIBerate-CVD and LIBerate-HR trials. We report safety and efficacy for a further 72 weeks from the open label extension trial (LIBerate-OLE), in subjects continuing on lerodalcibep, or switched from placebo to lerodalcibep - Figure 1. Methods: Of 1655 (89.8%) patients, 1096 on lerodalcibep, 559 on placebo, who completed week 52, 1486 (90%) continued in the OLE. Participants received lerodalcibep 300 mg monthly immediately on, or within 28 days of, completion of the base trial and were dosed in clinic for 3 months after which they were able to self-dose and return to clinic every 3 months. The primary objectives of this study were to assess the long-term safety, tolerability, and efficacy after 48 and 72 weeks. Results: Mean age (range) was 63.9 (25 to 89) years, 37% female, 79% white, 18% black/multiracial, with 85.5% on statins, 18% on ezetimibe and 67% with CVD and 33% at very high or high risk for CVD. Mean (SD) baseline LDL-C 109 (40.7) mg/dL. Mean (SD) LDL-C reductions in the OLE from original baseline (Figure 2) were 61.8 (26.2)% and 63.6 (29.3)% at weeks 48 and 72 respectively with median LDL-C reduction 71% at week 72. The 2/3rds of patients treated with lerodalcibep for 52 weeks in the base trial plus 72 weeks in OLE showed consistent mean reductions in excess of 60% throughout the >2 years of treatment with no signs of attenuation. During the OLE trial 93% of patients had an LDL-C reduction of >50%, 90% achieved their new lower risk-associated LDL-C target and 88% both goals – Figure 3. Reductions in mean (SD) Apo B and Non-HDL-C were 45 (21)% and 50.8 (22.7)% at week 48 and 44.9 (21.9)% and 53 (26.4)% week 72 respectively. Median (IQR) changes for Lp(a) were -30 (-11.8;-48.6)% at week 48 and -32.3 (-14.9;-50)% at week 72. Lerodalcibep was well tolerated, with no significant additional safety concerns seen. Conclusions: Lerodalcibep significantly and persistently reduced LDL-C with no attenuation in subjects with, or at very high or high risk for, CVD on maximally tolerated statin and other oral agents for >2 years with good adherence to self-dosing, tolerability and no new safety concerns identified. Lerodalcibep enabled nearly 90% of patients to achieve the new globally harmonized more stringent LDL-C goals.
More abstracts on this topic:
Access to Lipid-Lowering Therapies is Limited by Payer Coverage Restrictions and High Out-of-Pocket Costs on Medicare Prescription Drug Plans
Young Grant, Bansal Kannu, Riello Ralph, Faridi Kamil, Clark Katherine, Desai Nihar
An Economic Evaluation of Non-HDL-Cholesterol and Apolipoprotein B as Treatment Targets for Lipid-Lowering Therapy in Primary PreventionLuebbe Samuel, Wilkins John, Moran Andrew, Sniderman Allan, Kohli-lynch Ciaran