Final ID:
Resistance Exercise Therapy After COVID-19 Infection: a Randomized, Controlled Trial
Abstract Body (Do not enter title and authors here): Objective: To determine the effects of a resistance exercise intervention on exercise capacity in adults after COVID-19 infection. Secondary objectives included assessments of health status.
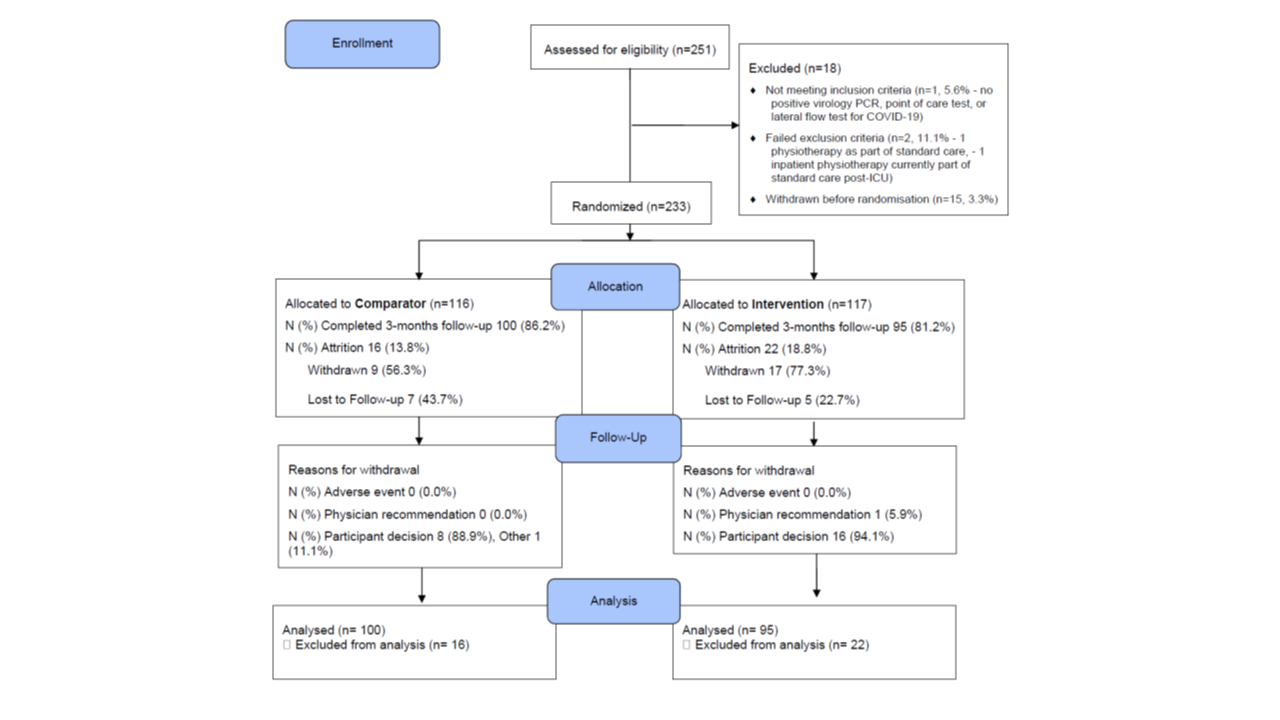
Study Design: A two-arm randomized, controlled clinical trial including adults with a hospital or community diagnosis of COVID-19 in the preceding 12 months was undertaken during June 2021-April 2024.
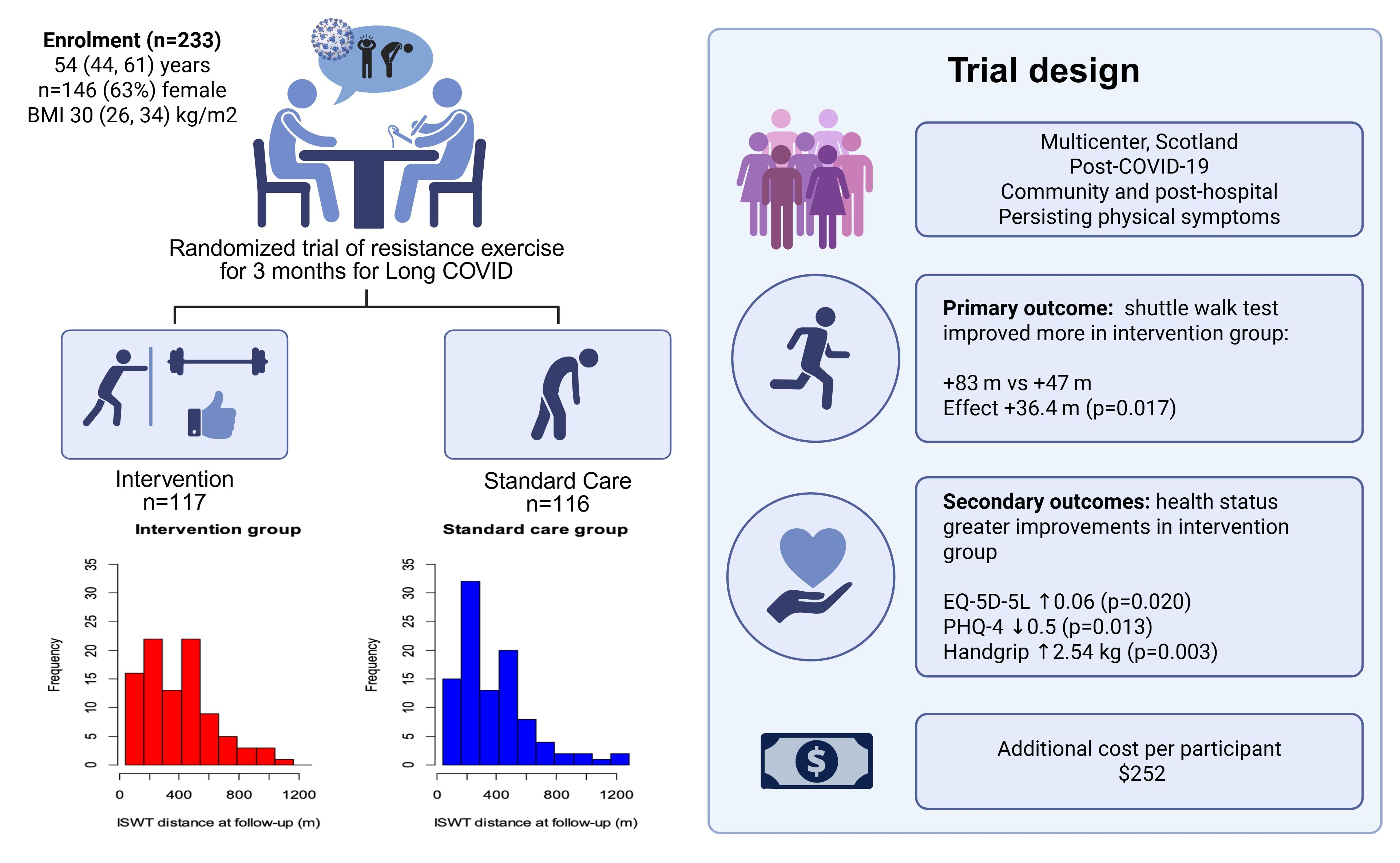
Population Studied: 233 individuals with persisting physical symptoms post-COVID-19.
Intervention: Personalized resistance exercise intervention for 3-months. A guideline was provided by research staff supported by an exercise physiologist. The document included an exercise log and links to training videos.
Control: Treatment-as-usual.
Power Calculation: Taking a clinically important between-group difference in the incremental shuttle walk test (ISWT) at follow-up (3-months) to be 46 m, with a SD of 105m, the sample size providing 80% power, at 5% significance, with no loss to follow-up (LTFU) was 85 per group; allowing for LTFU, the target was 110 per group (220 total).
Primary End Point: Distance achieved (m) in the ISWT at 3-months.
Secondary End Points: Health-related quality of life (EQ-5D-5L), anxiety and depression (Patient Health Questionnaire) and grip strength (kg).
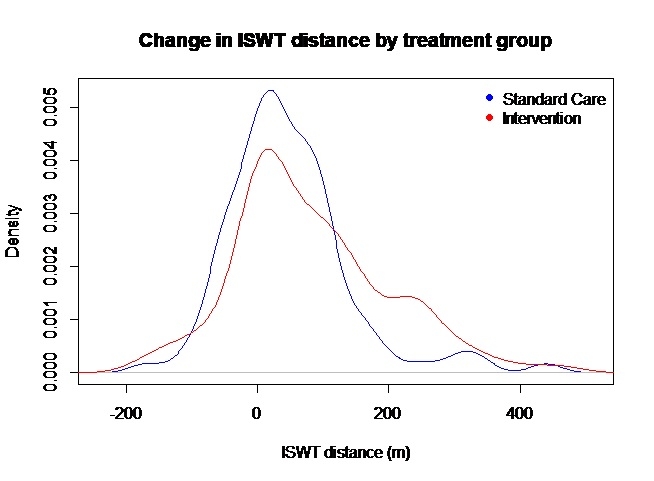
Outcome: 233 adults (median (interquartile range) 53.6 (43.8, 60.8) years; 146 (62.7%) female, 91 (39.1%) hospitalized with COVID-19 were randomized (n=117 (50.2%) intervention, n=116 (49.8%) control). The median (interquartile range) % adherence with the exercise intervention was 71.0 (47.8, 96.8), equivalent of performing exercises on 5 days/week. The mean (SD) distances achieved in the ISWT at baseline and at 3-months follow-up were 328 (225) m and 389 (249) m, in 224 and 193 individuals, respectively. The change in ISWT at 3-months compared to baseline was 83 (118) m in the intervention group (n=94) and 47 (95) m in the control group (n=98) (effect estimate (95%) confidence interval 36.5 (6.6, 66.3) m; p=0.017).
Greater improvements in the intervention group were observed for the EQ-5D-5L utility score (0.06 (0.01, 0.11); p=0.018), Patient Health Questionnaire (0.5 (0.2, 0.8); p=0.013) and handgrip strength (2.58 (0.92, 4.24) kg; p=0.002). The cost of the intervention per participant was $252.
Conclusion: In this randomized clinical trial, a program of resistance exercise for 3 months in adults after COVID-19 infection, improved exercise capacity, quality of life, anxiety and depression, and grip strength.
Study Design: A two-arm randomized, controlled clinical trial including adults with a hospital or community diagnosis of COVID-19 in the preceding 12 months was undertaken during June 2021-April 2024.
Population Studied: 233 individuals with persisting physical symptoms post-COVID-19.
Intervention: Personalized resistance exercise intervention for 3-months. A guideline was provided by research staff supported by an exercise physiologist. The document included an exercise log and links to training videos.
Control: Treatment-as-usual.
Power Calculation: Taking a clinically important between-group difference in the incremental shuttle walk test (ISWT) at follow-up (3-months) to be 46 m, with a SD of 105m, the sample size providing 80% power, at 5% significance, with no loss to follow-up (LTFU) was 85 per group; allowing for LTFU, the target was 110 per group (220 total).
Primary End Point: Distance achieved (m) in the ISWT at 3-months.
Secondary End Points: Health-related quality of life (EQ-5D-5L), anxiety and depression (Patient Health Questionnaire) and grip strength (kg).
Outcome: 233 adults (median (interquartile range) 53.6 (43.8, 60.8) years; 146 (62.7%) female, 91 (39.1%) hospitalized with COVID-19 were randomized (n=117 (50.2%) intervention, n=116 (49.8%) control). The median (interquartile range) % adherence with the exercise intervention was 71.0 (47.8, 96.8), equivalent of performing exercises on 5 days/week. The mean (SD) distances achieved in the ISWT at baseline and at 3-months follow-up were 328 (225) m and 389 (249) m, in 224 and 193 individuals, respectively. The change in ISWT at 3-months compared to baseline was 83 (118) m in the intervention group (n=94) and 47 (95) m in the control group (n=98) (effect estimate (95%) confidence interval 36.5 (6.6, 66.3) m; p=0.017).
Greater improvements in the intervention group were observed for the EQ-5D-5L utility score (0.06 (0.01, 0.11); p=0.018), Patient Health Questionnaire (0.5 (0.2, 0.8); p=0.013) and handgrip strength (2.58 (0.92, 4.24) kg; p=0.002). The cost of the intervention per participant was $252.
Conclusion: In this randomized clinical trial, a program of resistance exercise for 3 months in adults after COVID-19 infection, improved exercise capacity, quality of life, anxiety and depression, and grip strength.
More abstracts on this topic:
NICE-Support program effectively improves symptom and psychological distress in patients with heart failure
Liao Hui-ya, Chiou Ai-fu
Age-related Differences in Peak Oxygen Uptake in Patients with Multimorbidity Undergoing Cardiac RehabilitationGomes Pauline, Miller Sophie, Chacin-suarez Audry, Olson Thomas