Final ID: Sa3072
Obicetrapib in Patients with Dyslipidemia with or without Atherosclerotic Cardiovascular Disease Risk: A Meta-Analysis of Randomized Controlled Trials
Abstract Body (Do not enter title and authors here): Background: Guideline-directed lipid-lowering still leaves up to one-third of high-risk patients above LDL-C targets, sustaining residual cardiovascular risk. Obicetrapib—an oral cholesteryl-ester transfer-protein inhibitor has produced promising results in LDL-C reductions in recent phase 2/3 trials. We aim to quantify the efficacy and safety of Obicetrapib versus placebo in statin-treated adults.
Methods: We searched PubMed, Embase, Cochrane, Scopus, and Web of Science (inception to 15 May 2025) for randomised controlled trials (RCTs) that enrolled adults with dyslipidemia (with or without atherosclerotic cardiovascular disease risk (ASCVD) receiving statins and compared Obicetrapib (1, 2.5, 5, or 10 mg) against placebo. Dichotomous outcomes were pooled as risk ratios (RRs), and continuous outcomes as mean differences (MDs), each with 95 % confidence intervals (CIs).
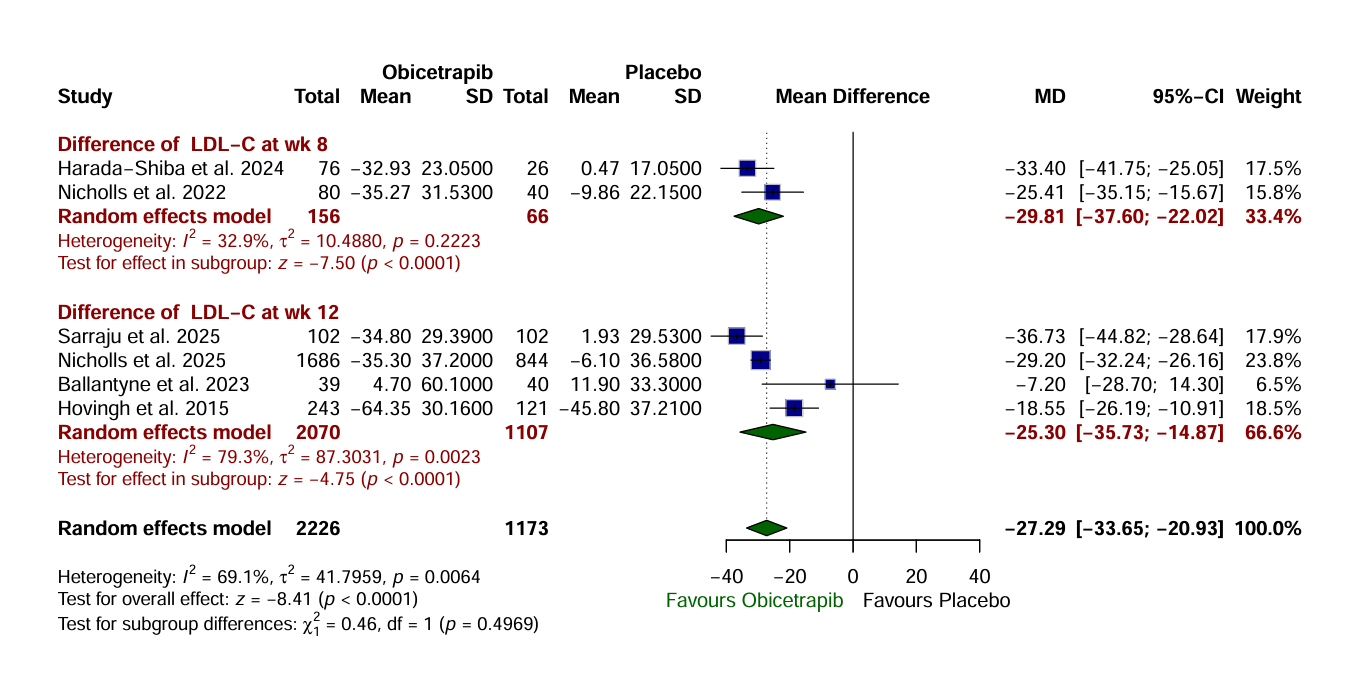
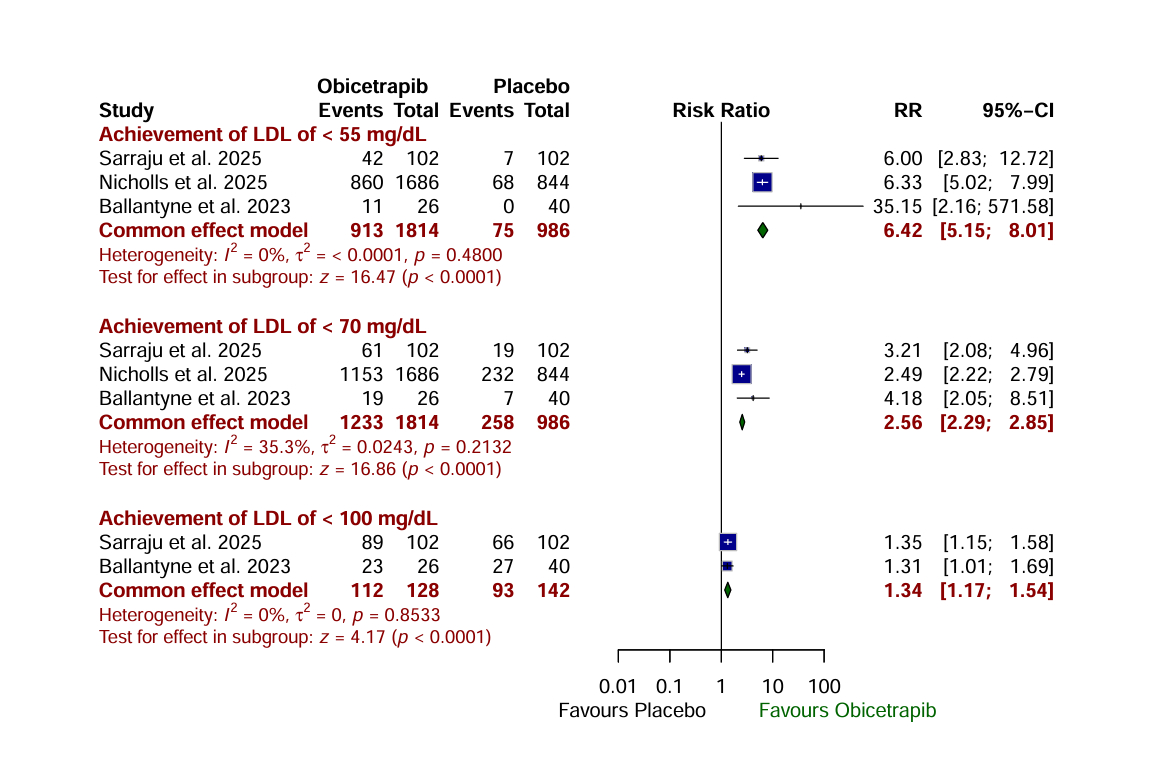
Results: A total of 3,386 patients were included across six RCTs. Obicetrapib, compared to placebo, was associated with a significantly greater reduction in LDL-C (MD: -27.29 mg/dL; 95% CI: -33.65 to -20.93; P < 0.0001) and a significantly greater increase in HDL-C (MD: 70.96 mg/dL; 95% CI: 64.71 to 77.20; P < 0.0001). Obicetrapib also significantly increased the likelihood of achieving LDL-C targets of <55 mg/dL (RR: 6.42; 95% CI: 5.15 to 8.01; P < 0.0001), <70 mg/dL (RR: 2.56; P < 0.0001), and <100 mg/dL (RR: 1.34; P < 0.0001). Additionally, apolipoprotein B levels were significantly reduced with Obicetrapib (MD: -14.34 mg/dL; 95% CI: -19.28 to -9.41; P < 0.0001). Additionally, there was no significant difference in total adverse events (AEs) (P= 0.4124), serious AEs (P= 0.3712), and AEs leading to discontinuation of medication (P=0.4035).
Conclusion: Our analysis shows that Obicetrapib, a selective CETP inhibitor, significantly improves lipid profiles in statin-treated patients with dyslipidemia, with or without ASCVD. It led to marked reductions in LDL-C, non-HDL-C, and apoB, and substantial increases in HDL-C. These effects significantly increased the achievement of LDL-C targets. Obicetrapib’s safety profile was similar to that of the placebo. Further RCTs are needed to confirm these findings.
Methods: We searched PubMed, Embase, Cochrane, Scopus, and Web of Science (inception to 15 May 2025) for randomised controlled trials (RCTs) that enrolled adults with dyslipidemia (with or without atherosclerotic cardiovascular disease risk (ASCVD) receiving statins and compared Obicetrapib (1, 2.5, 5, or 10 mg) against placebo. Dichotomous outcomes were pooled as risk ratios (RRs), and continuous outcomes as mean differences (MDs), each with 95 % confidence intervals (CIs).
Results: A total of 3,386 patients were included across six RCTs. Obicetrapib, compared to placebo, was associated with a significantly greater reduction in LDL-C (MD: -27.29 mg/dL; 95% CI: -33.65 to -20.93; P < 0.0001) and a significantly greater increase in HDL-C (MD: 70.96 mg/dL; 95% CI: 64.71 to 77.20; P < 0.0001). Obicetrapib also significantly increased the likelihood of achieving LDL-C targets of <55 mg/dL (RR: 6.42; 95% CI: 5.15 to 8.01; P < 0.0001), <70 mg/dL (RR: 2.56; P < 0.0001), and <100 mg/dL (RR: 1.34; P < 0.0001). Additionally, apolipoprotein B levels were significantly reduced with Obicetrapib (MD: -14.34 mg/dL; 95% CI: -19.28 to -9.41; P < 0.0001). Additionally, there was no significant difference in total adverse events (AEs) (P= 0.4124), serious AEs (P= 0.3712), and AEs leading to discontinuation of medication (P=0.4035).
Conclusion: Our analysis shows that Obicetrapib, a selective CETP inhibitor, significantly improves lipid profiles in statin-treated patients with dyslipidemia, with or without ASCVD. It led to marked reductions in LDL-C, non-HDL-C, and apoB, and substantial increases in HDL-C. These effects significantly increased the achievement of LDL-C targets. Obicetrapib’s safety profile was similar to that of the placebo. Further RCTs are needed to confirm these findings.
More abstracts on this topic:
Blood Lead Levels and Their Association with Cardiometabolic Outcomes in Adolescents: Preliminary Findings from the National ECHO Cohort
Idris Muhammed, Johnson Jabril
Association of Increased AST/ALT Ratio with Future Cardiovascular Events in Diabetic Patients without Prior Cardiovascular DiseaseOno Yoshiyasu, Ikeda Shota, Shinohara Keisuke, Matsumoto Sho, Yoshida Daisuke, Nakashima Ryosuke, Nakashima Hiroka, Miyamoto Ryohei, Abe Kohtaro