Final ID: MP817
Right Ventricular Free Wall Strain and Clinical Outcomes in Transthyretin Amyloid Cardiomyopathy and Effect of Vutrisiran: the HELIOS-B Study
Abstract Body (Do not enter title and authors here): Introduction: Right ventricular dysfunction is common among patients with transthyretin amyloid cardiomyopathy (ATTR-CM) and is associated with worse prognosis. In HELIOS-B, vutrisiran reduced rates of all-cause mortality (ACM) and recurrent cardiovascular (CV) events among patients with ATTR-CM compared with placebo and had beneficial effects on cardiac structure and function. Its effects on right ventricular free wall strain (RVFWS) are unknown.
Hypotheses: RVFWS is associated with clinical outcomes among patients with ATTR-CM. Vutrisiran has favorable effects on RVFWS.
Methods: HELIOS-B randomized 655 patients with ATTR-CM to vutrisiran (25mg subcutaneously every 12 weeks) or placebo. Echocardiograms were performed serially during follow-up. The association of baseline RVFWS with ACM and recurrent CV events was investigated using a modified Andersen-Gill model, adjusted for age, sex, ATTR disease type, National Amyloidosis Centre (NAC) stage, RV fractional area change (FAC), and tricuspid annular systolic myocardial velocity (RV S’), and stratified by baseline tafamidis use and treatment assignment. Changes in RVFWS from baseline to month 30 were evaluated using linear regression, adjusted for baseline RVFWS and clinical characteristics.
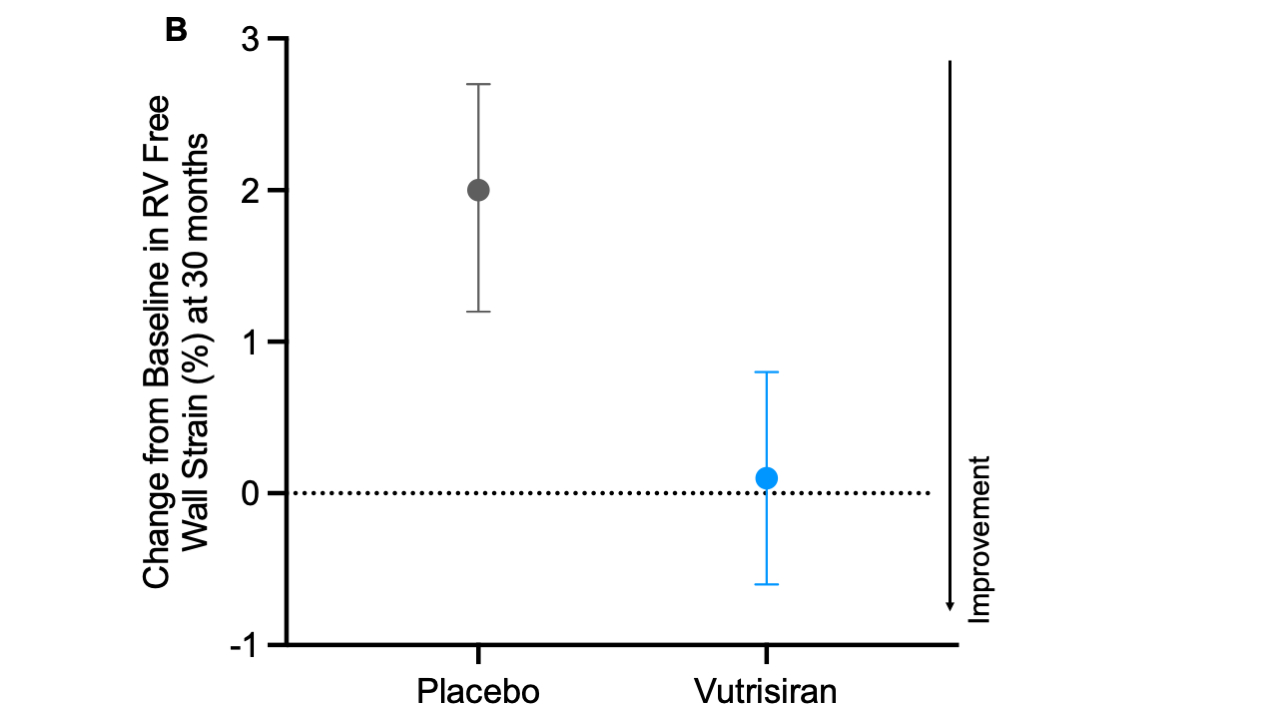
Results: Among 548 (84%) patients with available baseline RVFWS (age 75 ± 7 years, 92% men, 88% wild-type ATTR), mean RVFWS was -14.5± 5.1%. RV dysfunction was prevalent in a greater proportion of patients using RVFWS (>-20%, 85%) as compared with RV S’ (<9.5cm/s, 54%) or RV FAC (<35%, 27%). Patients in the worst RVFWS quartile (>-10.9%, n=137) had more atrial fibrillation, lower eGFR, lower LVEF and worse NYHA class and NAC disease stage. Worse RVFWS at baseline was associated with a heightened risk of ACM and recurrent CV events (adjusted RR 1.38, 95% CI: 1.17 - 1.63), independent of demographic characteristics, ATTR disease type, NAC stage and non-deformation-based metrics of RV function. At 30 months, RVFWS remained stable in the vutrisiran group (0.1%, 95% CI: -0.6, 0.8%) and declined in the placebo group (2.0%, 95% CI:1.2, 2.7), between group difference (-1.6%, 95% CI: -2.6, -0.7%).
Conclusions: RVFWS is markedly impaired among patients with ATTR-CM and is strongly and independently associated with higher risk of ACM and recurrent CV events. Consistent with its beneficial effects on other measures of cardiac structure and function, vutrisiran stabilized RVFWS at 30 months compared with worsening in the placebo group.
Hypotheses: RVFWS is associated with clinical outcomes among patients with ATTR-CM. Vutrisiran has favorable effects on RVFWS.
Methods: HELIOS-B randomized 655 patients with ATTR-CM to vutrisiran (25mg subcutaneously every 12 weeks) or placebo. Echocardiograms were performed serially during follow-up. The association of baseline RVFWS with ACM and recurrent CV events was investigated using a modified Andersen-Gill model, adjusted for age, sex, ATTR disease type, National Amyloidosis Centre (NAC) stage, RV fractional area change (FAC), and tricuspid annular systolic myocardial velocity (RV S’), and stratified by baseline tafamidis use and treatment assignment. Changes in RVFWS from baseline to month 30 were evaluated using linear regression, adjusted for baseline RVFWS and clinical characteristics.
Results: Among 548 (84%) patients with available baseline RVFWS (age 75 ± 7 years, 92% men, 88% wild-type ATTR), mean RVFWS was -14.5± 5.1%. RV dysfunction was prevalent in a greater proportion of patients using RVFWS (>-20%, 85%) as compared with RV S’ (<9.5cm/s, 54%) or RV FAC (<35%, 27%). Patients in the worst RVFWS quartile (>-10.9%, n=137) had more atrial fibrillation, lower eGFR, lower LVEF and worse NYHA class and NAC disease stage. Worse RVFWS at baseline was associated with a heightened risk of ACM and recurrent CV events (adjusted RR 1.38, 95% CI: 1.17 - 1.63), independent of demographic characteristics, ATTR disease type, NAC stage and non-deformation-based metrics of RV function. At 30 months, RVFWS remained stable in the vutrisiran group (0.1%, 95% CI: -0.6, 0.8%) and declined in the placebo group (2.0%, 95% CI:1.2, 2.7), between group difference (-1.6%, 95% CI: -2.6, -0.7%).
Conclusions: RVFWS is markedly impaired among patients with ATTR-CM and is strongly and independently associated with higher risk of ACM and recurrent CV events. Consistent with its beneficial effects on other measures of cardiac structure and function, vutrisiran stabilized RVFWS at 30 months compared with worsening in the placebo group.
More abstracts on this topic:
A Phase I, Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Single Ascending Doses of N-acetylgalactosamine Small Interfering RNA Conjugate, BPR-30221616, in Healthy Participants, for Potential Treatment of Transthyretin Amyloidosis
Han Xiaohong, Chen Rui, Cheng Xiwen, Zhang Langxi, Huang Haoxi, Fan Shengjun
A Diagnostic Challenge: Wild-Type Transthyretin Cardiac Amyloidosis in a Patient With Systemic Lupus and Ischemic CardiomyopathyAbdallah Ala, Khalid Arbab, Dicaro Michael, Lei Kachon, Ahsan Chowdhury