Final ID: 4171680
Impact of vutrisiran on outpatient worsening heart failure in patients with transthyretin amyloidosis with cardiomyopathy in the HELIOS-B trial
Abstract Body (Do not enter title and authors here): Background: Transthyretin amyloidosis with cardiomyopathy (ATTR-CM) is a fatal disease, caused by transthyretin amyloid fibril deposits in the heart. Practical and sensitive methods are needed to monitor patients with disease progression and optimize treatment decisions. Outpatient worsening heart failure (HF) (oral diuretic intensification or initiation) has been shown to be prognostic of mortality in patients with ATTR-CM. In the phase 3 HELIOS-B trial (NCT04153149), the RNAi therapeutic vutrisiran reduced the risk of all-cause mortality (ACM) and recurrent CV events (CV hospitalizations and urgent HF visits) vs placebo in patients with ATTR-CM.
Aims: To investigate the clinical and prognostic value of – in addition to the effect of vutrisiran on – outpatient worsening HF in patients with ATTR-CM.
Methods: Associations between outpatient worsening HF and the HELIOS-B primary composite of ACM and recurrent CV events, ACM alone, and other disease progression-related endpoints were evaluated. The impact of vutrisiran over 36 months on outpatient worsening HF and an expanded composite of ACM, recurrent CV events, and outpatient worsening HF was also assessed.
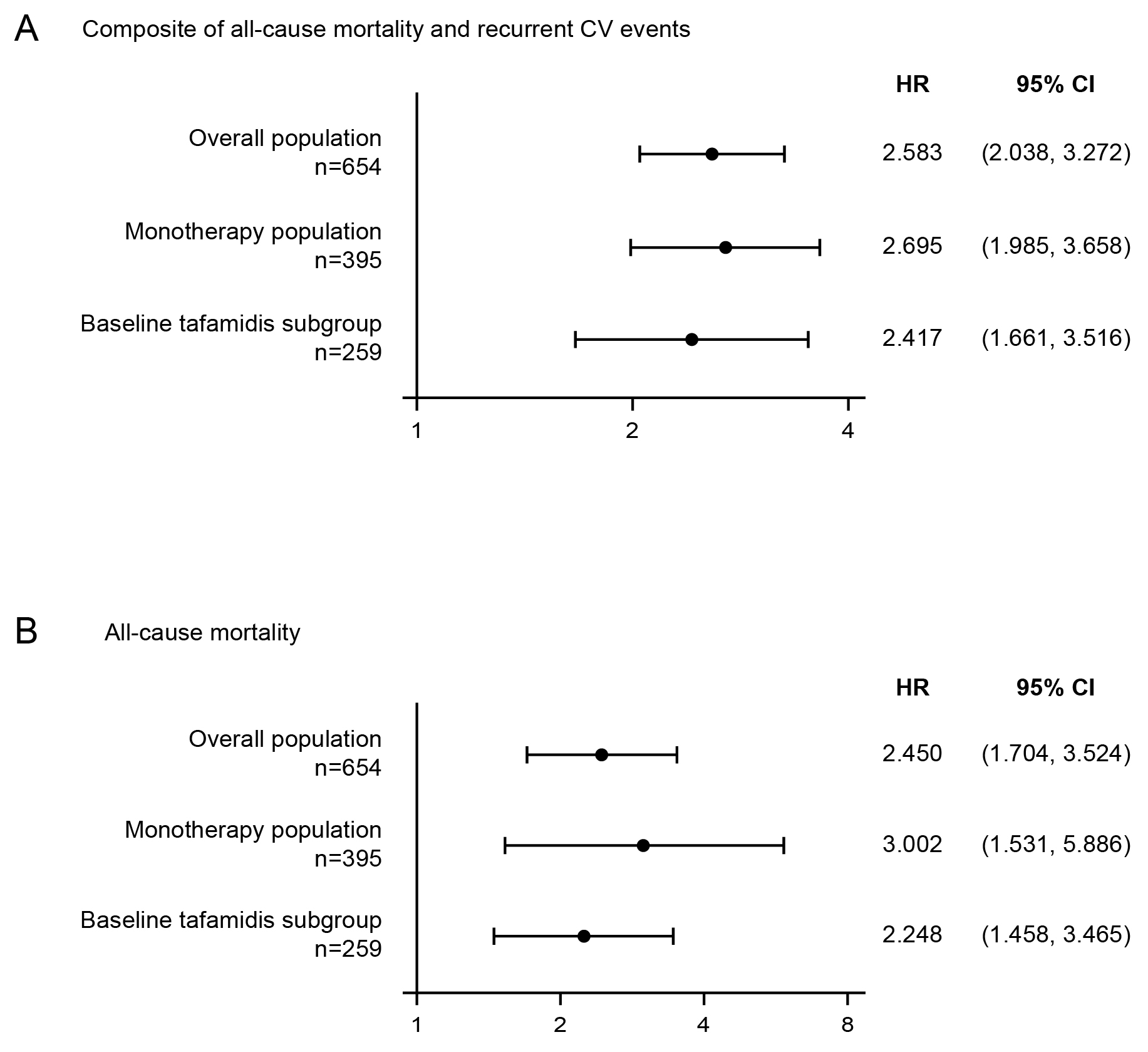
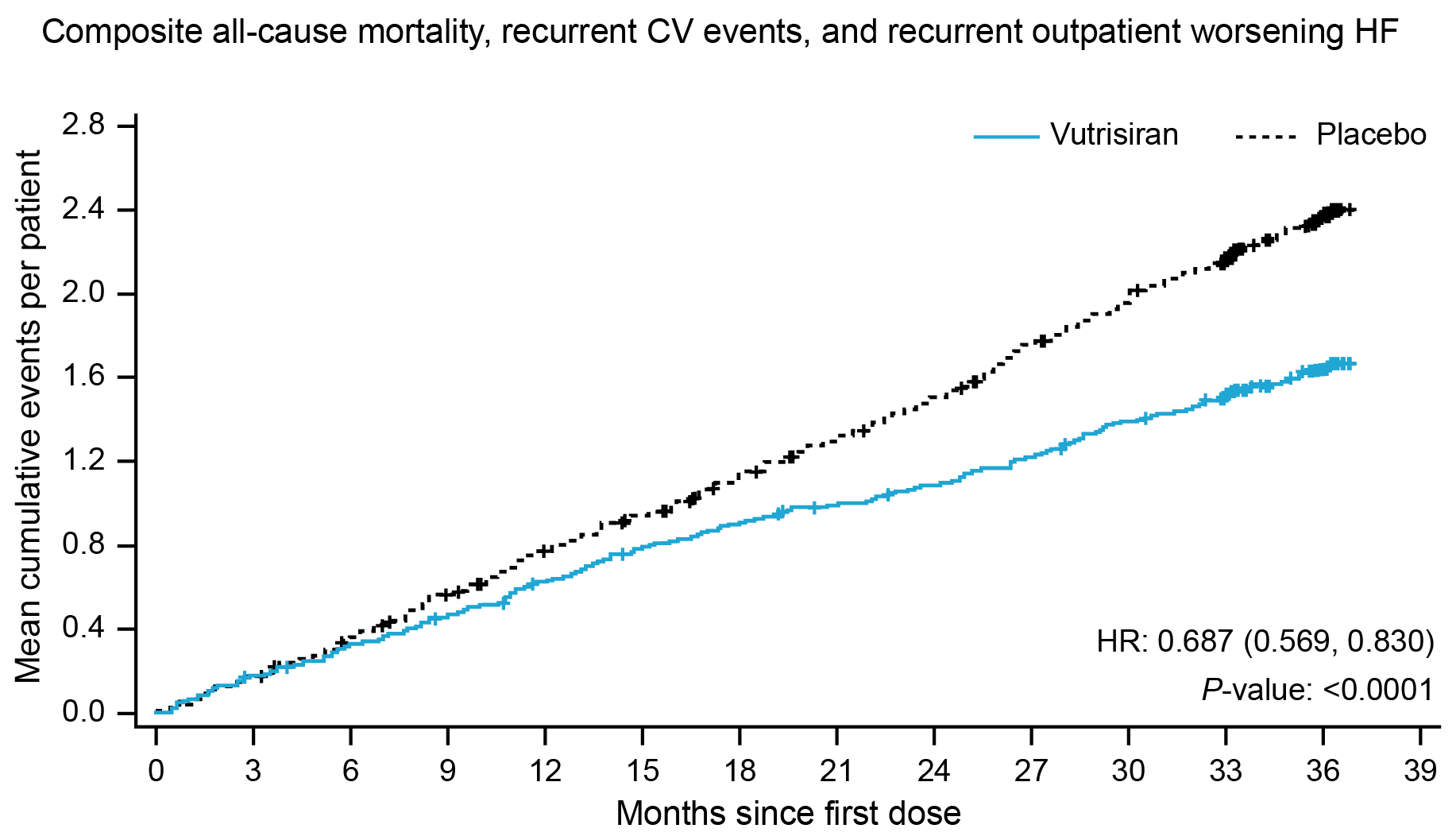
Results: In the overall population (n=655 randomized; n=654 treated), 321 (49.1%) patients had ≥1 outpatient worsening HF event, 245 (37.5%) had ≥1 CV event(s), and 120 (18.3%) died; 237 patients (36.2%) had no events. Patients with, vs those without, outpatient worsening HF had an increased risk of ACM and CV events (hazard ratio [HR] 2.58, 95% confidence interval [CI] 2.04, 3.27) and ACM (HR 2.45, 95% CI 1.70, 3.52) (Figure 1), as well as greater deterioration in 6-minute walk test distance and Kansas City Cardiomyopathy Questionnaire-Overall Summary score, and a greater increase in N-terminal prohormone of B-type natriuretic peptide. In recurrent event analyses over the double-blind period, vutrisiran reduced the rate of outpatient worsening HF (relative rate ratio 0.66, 95% CI 0.56, 0.78) vs placebo. Vutrisiran also reduced the risk of the composite of ACM, recurrent CV events and outpatient worsening HF vs placebo (HR 0.69 [95% CI 0.57, 0.83]) (Figure 2).
Conclusions: Outpatient worsening HF was frequent in patients with ATTR-CM and was associated with an increased risk of mortality and recurrent CV events. Vutrisiran reduced the risk of outpatient worsening HF vs placebo.
Aims: To investigate the clinical and prognostic value of – in addition to the effect of vutrisiran on – outpatient worsening HF in patients with ATTR-CM.
Methods: Associations between outpatient worsening HF and the HELIOS-B primary composite of ACM and recurrent CV events, ACM alone, and other disease progression-related endpoints were evaluated. The impact of vutrisiran over 36 months on outpatient worsening HF and an expanded composite of ACM, recurrent CV events, and outpatient worsening HF was also assessed.
Results: In the overall population (n=655 randomized; n=654 treated), 321 (49.1%) patients had ≥1 outpatient worsening HF event, 245 (37.5%) had ≥1 CV event(s), and 120 (18.3%) died; 237 patients (36.2%) had no events. Patients with, vs those without, outpatient worsening HF had an increased risk of ACM and CV events (hazard ratio [HR] 2.58, 95% confidence interval [CI] 2.04, 3.27) and ACM (HR 2.45, 95% CI 1.70, 3.52) (Figure 1), as well as greater deterioration in 6-minute walk test distance and Kansas City Cardiomyopathy Questionnaire-Overall Summary score, and a greater increase in N-terminal prohormone of B-type natriuretic peptide. In recurrent event analyses over the double-blind period, vutrisiran reduced the rate of outpatient worsening HF (relative rate ratio 0.66, 95% CI 0.56, 0.78) vs placebo. Vutrisiran also reduced the risk of the composite of ACM, recurrent CV events and outpatient worsening HF vs placebo (HR 0.69 [95% CI 0.57, 0.83]) (Figure 2).
Conclusions: Outpatient worsening HF was frequent in patients with ATTR-CM and was associated with an increased risk of mortality and recurrent CV events. Vutrisiran reduced the risk of outpatient worsening HF vs placebo.
More abstracts on this topic:
A Case of Concomitant Wild-Type Transthyretin and Systemic Light Chain Amyloidosis Involving Separate Organs
Chiu Leonard, Afrough Aimaz, Nadeem Urooba, Jebakumar Deborah, Grodin Justin
Combinatorial Transcription Factor Gene Transfer Accelerates Direct Reprogramming of Ventricular Myocytes to Pacemaker PhenotypeFan Jinqi, Leng Jing, Cho Hee Cheol