Final ID: MP816
Left Atrial Reservoir Strain and Clinical Outcomes in Transthyretin Amyloid Cardiomyopathy: Insights from the HELIOS-B Trial on Vutrisiran Efficacy
Abstract Body (Do not enter title and authors here): Introduction: Amyloid infiltration of the atria and cardiac remodeling in response to elevations in intracardiac filling pressures lead to atrial dysfunction in patients with transthyretin amyloid cardiomyopathy (ATTR-CM). In the HELIOS-B trial, vutrisiran reduced rates of all-cause mortality and recurrent cardiovascular (CV) events among patients with ATTR-CM and had favorable effects on cardiac structure and ventricular function. The effects of vutrisiran on left atrial reservoir strain (LASr), a measure of left atrial function, have not been described.
Hypotheses: LASr is prognostically important in patients with ATTR-CM. Vutrisiran has favorable effects on LASr.
Methods: In the HELIOS-B trial, 655 patients with ATTR-CM were randomized to receive vutrisiran (25 mg subcutaneously every 12 weeks) versus placebo. Echocardiograms were performed serially throughout the trial. Associations of baseline LASr with all-cause mortality as well as all-cause mortality and recurrent CV events were evaluated using Poisson regression models adjusted for age, ATTR disease type, National Amyloidosis Centre (NAC) disease stage, atrial fibrillation/flutter, left atrial volume index, LVEF, baseline tafamidis use and treatment assignment. Changes in LASr from baseline to month 30 were assessed using linear regression, adjusted for baseline LASr and relevant clinical covariates.
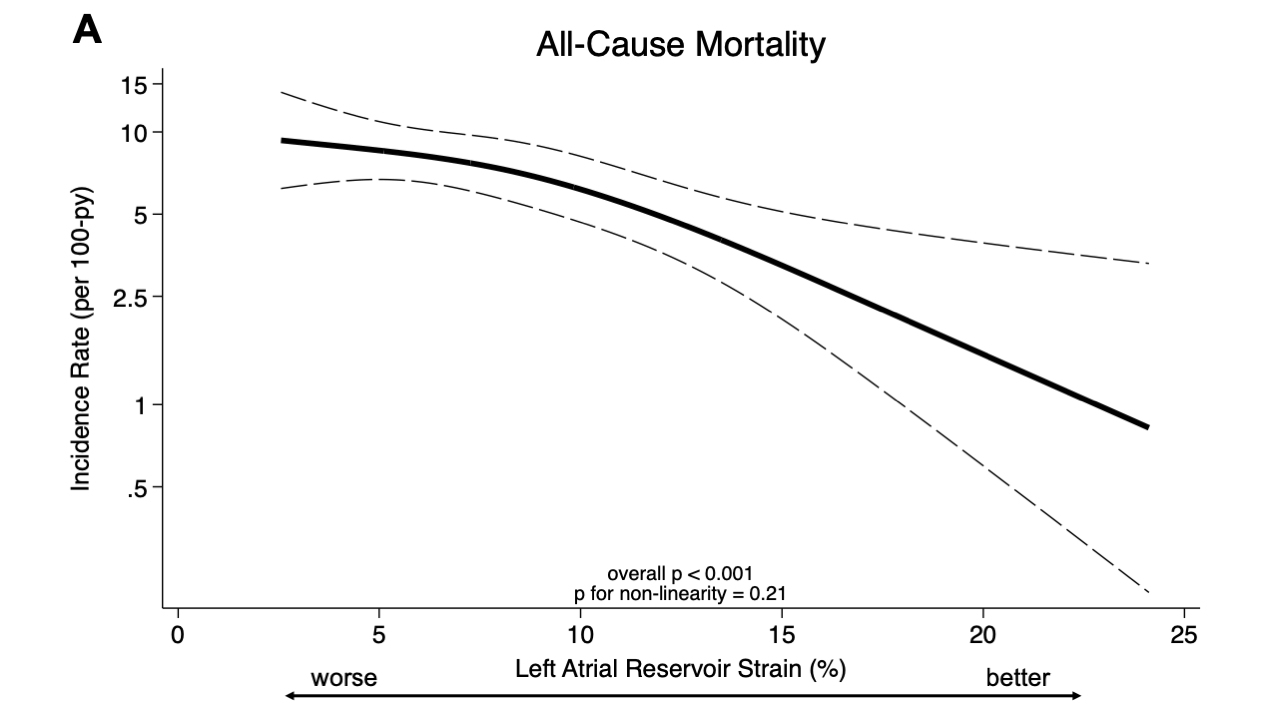
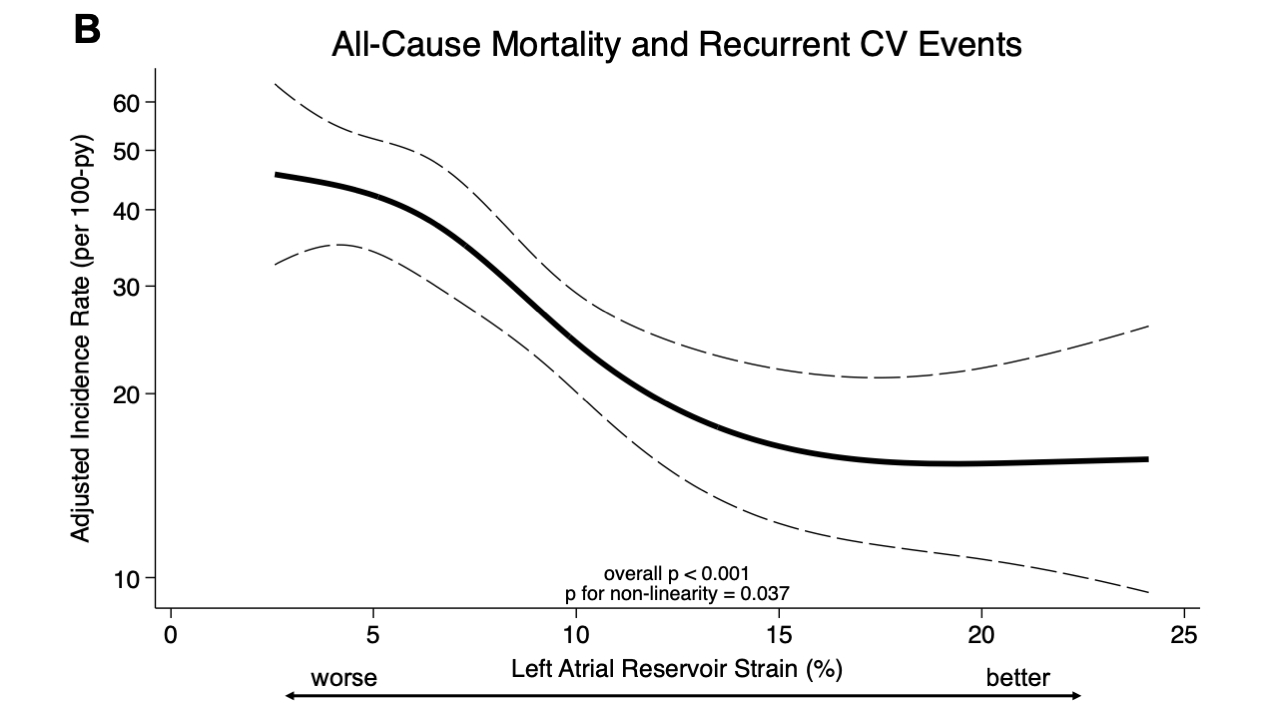
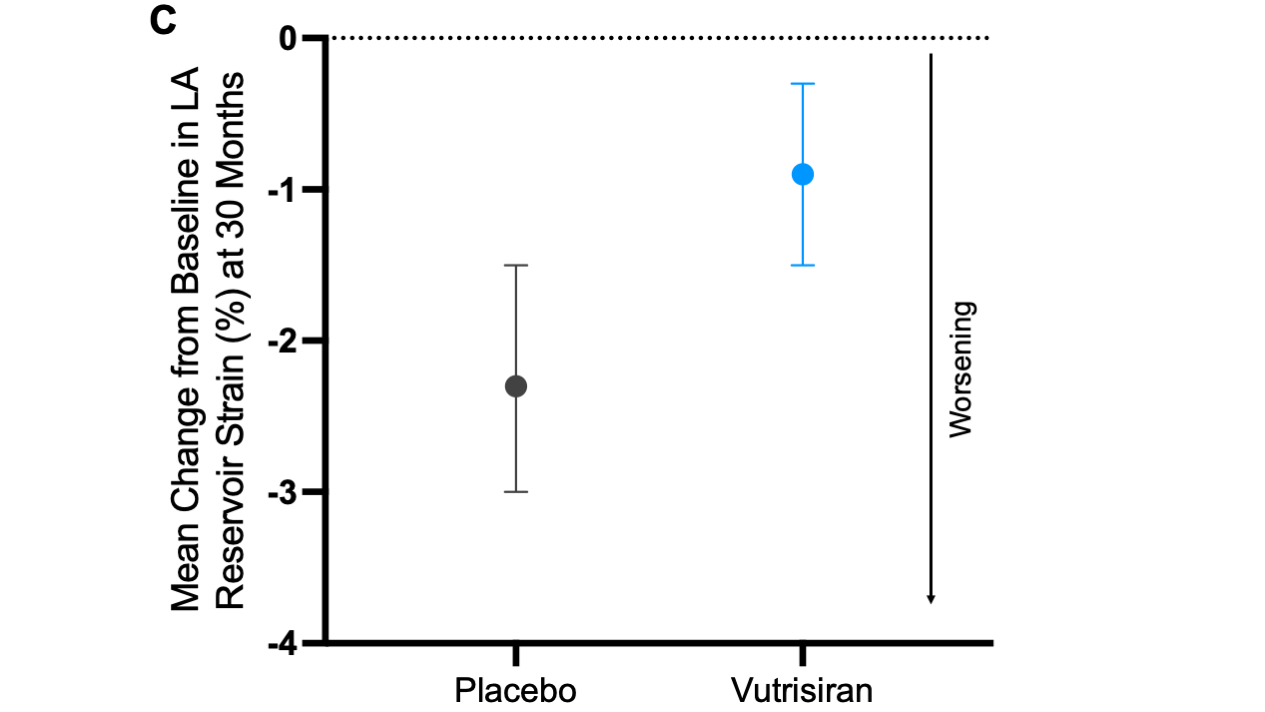
Results: Among the 644 (98%) patients with available baseline LASr (mean age 75 ± 7 years, 93% men, 88% wild-type ATTR), median LASr was 8.2% [IQR 5.4-12.1%]. Patients with worse LASr were more frequently men, had more atrial fibrillation and worse eGFR, LVEF, NAC disease stage and NYHA functional class. Worse LASr was independently associated with a greater risk of all-cause mortality as well as all-cause mortality and recurrent CV events (Panels A-B). At 30 months, LASr worsened less in the vutrisiran group (-0.9%, 95% CI: -1.5, -0.3%) compared with the placebo group (-2.3%, 95% CI: -3.0, -1.5%) with a between group difference of 1.2% (95% CI: 0.4-2.0%) (Panel C).
Conclusions: Impairment in LASr is common among patients with ATTR-CM and is significantly and independently associated with all-cause mortality and recurrent CV events. Consistent with its beneficial effects on other measures of cardiac structure and function, vutrisiran attenuated worsening in LASr at 30 months compared with placebo. These findings support the central role of LA function in the pathophysiology of ATTR-CM.
Hypotheses: LASr is prognostically important in patients with ATTR-CM. Vutrisiran has favorable effects on LASr.
Methods: In the HELIOS-B trial, 655 patients with ATTR-CM were randomized to receive vutrisiran (25 mg subcutaneously every 12 weeks) versus placebo. Echocardiograms were performed serially throughout the trial. Associations of baseline LASr with all-cause mortality as well as all-cause mortality and recurrent CV events were evaluated using Poisson regression models adjusted for age, ATTR disease type, National Amyloidosis Centre (NAC) disease stage, atrial fibrillation/flutter, left atrial volume index, LVEF, baseline tafamidis use and treatment assignment. Changes in LASr from baseline to month 30 were assessed using linear regression, adjusted for baseline LASr and relevant clinical covariates.
Results: Among the 644 (98%) patients with available baseline LASr (mean age 75 ± 7 years, 93% men, 88% wild-type ATTR), median LASr was 8.2% [IQR 5.4-12.1%]. Patients with worse LASr were more frequently men, had more atrial fibrillation and worse eGFR, LVEF, NAC disease stage and NYHA functional class. Worse LASr was independently associated with a greater risk of all-cause mortality as well as all-cause mortality and recurrent CV events (Panels A-B). At 30 months, LASr worsened less in the vutrisiran group (-0.9%, 95% CI: -1.5, -0.3%) compared with the placebo group (-2.3%, 95% CI: -3.0, -1.5%) with a between group difference of 1.2% (95% CI: 0.4-2.0%) (Panel C).
Conclusions: Impairment in LASr is common among patients with ATTR-CM and is significantly and independently associated with all-cause mortality and recurrent CV events. Consistent with its beneficial effects on other measures of cardiac structure and function, vutrisiran attenuated worsening in LASr at 30 months compared with placebo. These findings support the central role of LA function in the pathophysiology of ATTR-CM.
More abstracts on this topic:
Abrupt cardiac rupture of the patient with ATTR amyloidosis
Tagata Kento, Yutaro Nomoto, Tao Koji, Kataoka Tetsuro, Ohishi Mitsuru
A refined definition for low-risk pulmonary arterial hypertension patients including mortality and morbidityFauvel Charles, Liu Yongqi, Correa-jaque Priscilla, Everett Allen, Kanwar Manreet, Vanderpool Rebecca, Sahay Sandeep, Lin Shili, Benza Raymond