Final ID: 55
A Novel Thrombolytic with Anti-inflammatory Properties (JX10) Improves Neurological Outcomes in Acute Lacunar Infarct up to 12 hours After Onset
Methods: JX10 or placebo was administered as a single intravenous infusion at a dose of 1, 3, or 6 mg/kg to AIS patients who were ineligible for tissue plasminogen activator or thrombectomy within 12 h of LKW. Safety and Efficacy outcomes were assessed at 90 days.
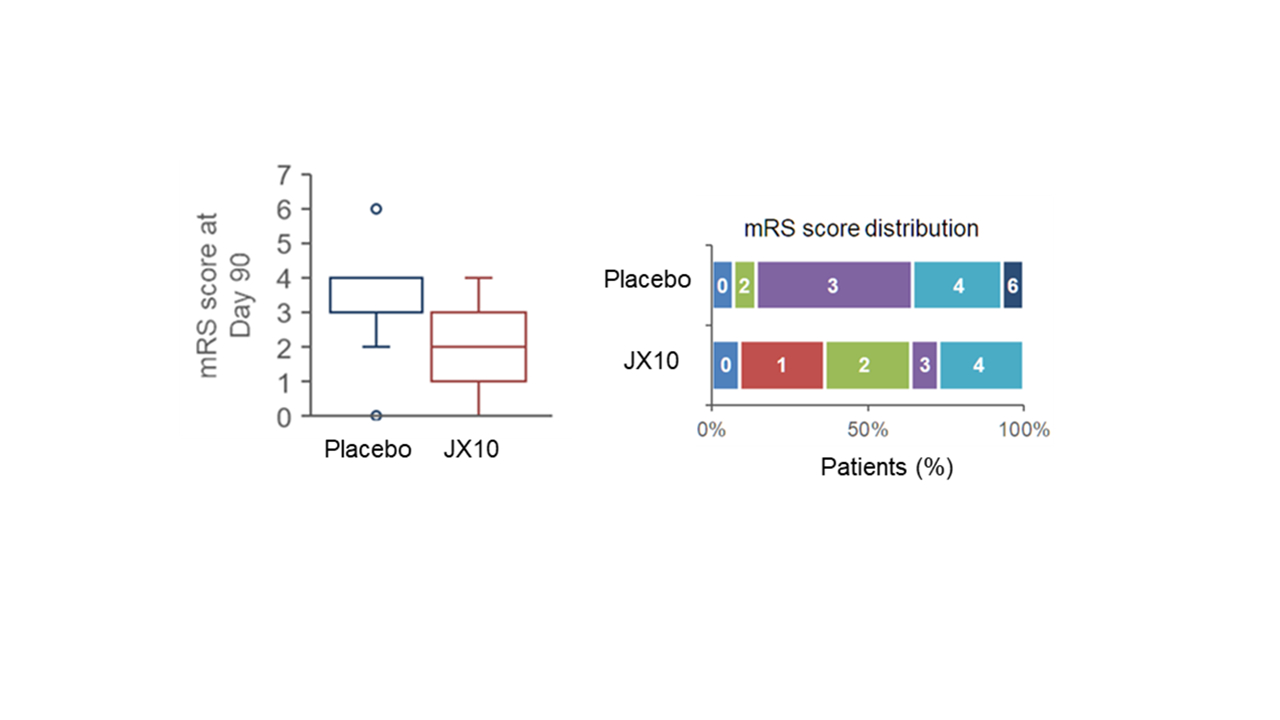
Results: Among the 90 patients enrolled in the trial, a total of 25 patients with acute lacunar infarct were dosed (JX10 1 mg/kg group: 1 subject; 3 mg/kg group: 3 subjects; 6 mg/kg group: 7 subjects; and placebo group: 14 subjects). In the JX10 1, 3, 6 mg/kg, pooled groups, and the placebo group, the rates of mRS 0–1 were 0 subject out of 1 (0.0%), 1 subject out of 3 (33.3%), 3 subjects out of 7 (42.9%), 4 subjects out of 11 (36.4%), and 1 subject out of 14 (7.1%), respectively, and those of mRS 0–2 were 0 subjects out of 1 (0.0%), 3 subjects out of 3 (100.0%), 4 subject out of 7 (57.1%), 7 subjects out of 11 (63.6%), and 2 subjects out of 14 (14.3%), respectively. Despite small numbers, patients with acute lacunar infarct who were treated with JX10 showed trend of improved neurologic function at 90 days, as measured by mRS. Symptomatic intracranial hemorrhage was not observed in any JX10 treated patients.
Conclusions: JX10 improved functional outcome in patients who presented with lacunar infarct, as measured by mRS at day 90 vs placebo. Findings support further testing of JX10 in larger and broader patient populations.
More abstracts on this topic:
Thomas Tarun, Tonetti Daniel, Shaikh Hamza, Jovin Tudor, Koneru Manisha, Dubinski Michael, Patel Karan, Penckofer Mary, Khalife Jane, Thon Jesse, Schumacher Hermann, Hanafy Khalid, Patel Pratit
A ChatGLM-based stroke diagnosis and prediction toolSong Xiaowei, Wang Jiayi, Ma Weizhi, Wu Jian, Wang Yueming, Gao Ceshu, Wei Chenming, Pi Jingtao
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.