Final ID: MP2773
Distinct Roles For CD36 Dependent Versus Independent Transendothelial Delivery And Uptake by Cardiomyocytes Are Revealed by Coordination With Vectorial Acylation by Esterification to Acyl-CoA.
Abstract Body (Do not enter title and authors here): Background: The heart primarily relies on long-chain fatty acids (LCFAs) for energy, utilizing endothelial (EC) and cardiomyocyte (CM) CD36 transporters for respective delivery to and uptake by CMs. While acyl-Coenzyme A synthetase 1 (ACSL1) facilitates LCFA uptake via vectorial acylation, a form of metabolic trapping, through esterification to acyl-CoA, the coordinated LCFA delivery and uptake by CMs with metabolic trapping by ACSL1 in CMs is unclear.
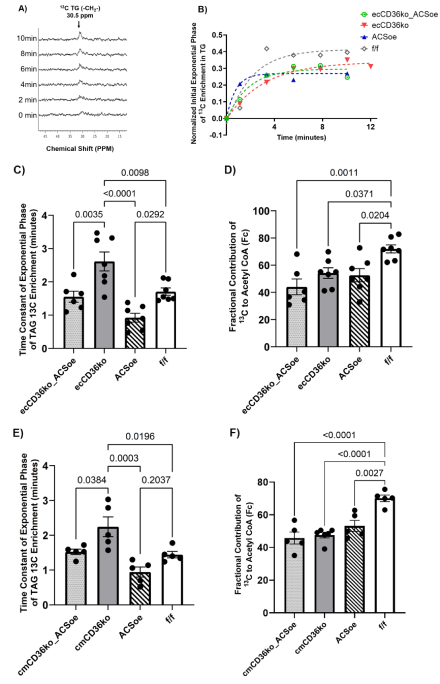
Methods: EC- and CM-specific CD36 knockout (KO) mice were crossed with cardiac specific, low ACSL1-overexpressing mice (MHC-ACSL1 J3). Isolated hearts were perfused with 13C palmitate/oleate, glucose and lactate. LCFA uptake kinetics were indexed in hearts by dynamic mode 13C NMR (Fig 1A,B). LCFA oxidation and metabolites were determined by end point NMR and mass spectrometry.
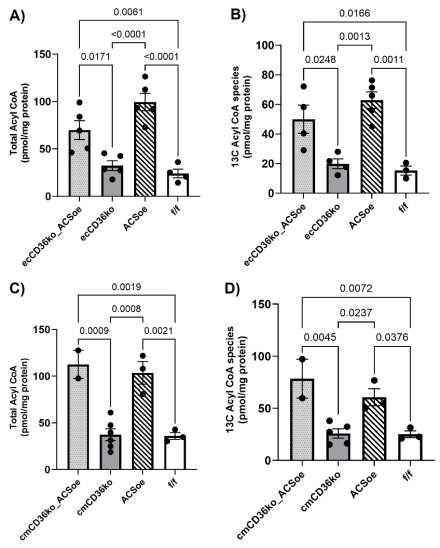
Results: EC- and CM-CD36 KO delayed LCFA uptake by CMs, as indexed by initial transport-dependent entry into naïve, unlabeled triglyceride (TG), highlighting distinct yet essential roles of each CD36 population (Fig 1B). Cardiac function was similar among groups. As published, MHC-ACSL1 alone reduced LCFA oxidation due to increased acyl-CoA, enabling substrate competition with high Km enzymes of other pathways. EC-CD36 KO delayed LCFA delivery to CMs, reducing LCFA entry into oxidative and cytosolic metabolism (Fc) (Fig 1C). CM-CD36 KO directly impaired LCFA entry into CMs, also reducing LCFA oxidation and cytosolic metabolism (Fig 1E). Metabolic trapping of LCFA by ACSL1 overexpression (ACSL1 OE) offset reduced LCFA entry rates into CD36 KO CMs (Fig 1D, 1F). ACSL1 OE increases metabolic trapping through increased acyl-CoA (Fig 2) and sustains LCFA gradients across the sarcolemma in EC-CD36 KO CMs to promote LCFA influx, inducing CD36-independent uptake.
Conclusion: 1) deleting CD36-dependent transendothelial delivery of LCFA to CMs slows, but does not eliminate uptake into CMs, leading to reduced LCFA oxidation and cytosolic metabolism, 2) the LCFA gradient across the sarcolemma in EC-CD36 KO can be increased by metabolic trapping via ACSL1 OE to restore LCFA uptake kinetics and cytosolic metabolism, 3) In CM-CD36KO hearts metabolic trapping of LCFA as acyl-CoA promotes uptake into CMs via CD36-independent mechanisms that restore LCFA metabolites, 4) Both EC- and CM-CD36 KO limit LCFA oxidation, which was not restored by high acyl-CoA due to substrate competition among higher Km enzymes.
Methods: EC- and CM-specific CD36 knockout (KO) mice were crossed with cardiac specific, low ACSL1-overexpressing mice (MHC-ACSL1 J3). Isolated hearts were perfused with 13C palmitate/oleate, glucose and lactate. LCFA uptake kinetics were indexed in hearts by dynamic mode 13C NMR (Fig 1A,B). LCFA oxidation and metabolites were determined by end point NMR and mass spectrometry.
Results: EC- and CM-CD36 KO delayed LCFA uptake by CMs, as indexed by initial transport-dependent entry into naïve, unlabeled triglyceride (TG), highlighting distinct yet essential roles of each CD36 population (Fig 1B). Cardiac function was similar among groups. As published, MHC-ACSL1 alone reduced LCFA oxidation due to increased acyl-CoA, enabling substrate competition with high Km enzymes of other pathways. EC-CD36 KO delayed LCFA delivery to CMs, reducing LCFA entry into oxidative and cytosolic metabolism (Fc) (Fig 1C). CM-CD36 KO directly impaired LCFA entry into CMs, also reducing LCFA oxidation and cytosolic metabolism (Fig 1E). Metabolic trapping of LCFA by ACSL1 overexpression (ACSL1 OE) offset reduced LCFA entry rates into CD36 KO CMs (Fig 1D, 1F). ACSL1 OE increases metabolic trapping through increased acyl-CoA (Fig 2) and sustains LCFA gradients across the sarcolemma in EC-CD36 KO CMs to promote LCFA influx, inducing CD36-independent uptake.
Conclusion: 1) deleting CD36-dependent transendothelial delivery of LCFA to CMs slows, but does not eliminate uptake into CMs, leading to reduced LCFA oxidation and cytosolic metabolism, 2) the LCFA gradient across the sarcolemma in EC-CD36 KO can be increased by metabolic trapping via ACSL1 OE to restore LCFA uptake kinetics and cytosolic metabolism, 3) In CM-CD36KO hearts metabolic trapping of LCFA as acyl-CoA promotes uptake into CMs via CD36-independent mechanisms that restore LCFA metabolites, 4) Both EC- and CM-CD36 KO limit LCFA oxidation, which was not restored by high acyl-CoA due to substrate competition among higher Km enzymes.
More abstracts on this topic:
A Metabolomic Study of Cardiac Dysfunction in Hyperglycemia
Yoshida Yilin, Qi Qibin, Cheng Susan, Kaplan Robert, Rodriguez Carlos, Shah Amil, Yu Bing, Nguyen Ngoc Quynh, Moon Eun Hye, Casey Rebholz, Skali Hicham, Arthur Victoria, Echouffo Justin, Ballantyne Christie, Selvin Elizabeth
A Mechanistic Insight Into The Connection Between Metabolism And Differentiation In ACTA2 P. R179 Smooth Muscle CellsEsparza Pinelo Jose, Krenz Hannah, Chen Jessica, Kaw Anita, Milewicz Dianna, Kwartler Callie