Final ID: MP2769
Persistent Metabolic Remodeling Following Alleviation of Afterload Stress in a Preclinical Model, Despite Recovery of Ejection Fraction and Left Ventricle Hypertrophy
Abstract Body (Do not enter title and authors here): Background and Hypothesis: Aortic stenosis (AS) causes afterload stress and left ventricular hypertrophy (LVH), treatable by aortic valve replacement (AVR) restoring ejection fraction (EF) and reducing LVH . Afterload stress also induces metabolic remodeling with reduced long chain fatty acid (LCFA) oxidation (FAO). This study examines a preclinical correlate of afterload stress and alleviation after LVH, dysfunction, and metabolic remodeling in mice with transverse aortic constriction (TAC) and release.
Methods: Adult male C57BL/6J mice underwent Sham surgery, TAC (11wks), or TAC-REM (TAC removal at 11wks with 3 wks recovery). In vivo function was monitored via echocardiography. At endpoint, isolated hearts were perfused with a physiological mix of 13C enriched LCFA, 13C-palmitate, with glucose and lactate. In vitro 13C NMR provided fractional contribution (Fc) to oxidative metabolism. Markers of LVH and metabolic remodeling were assessed by qPCR and immunoblot.
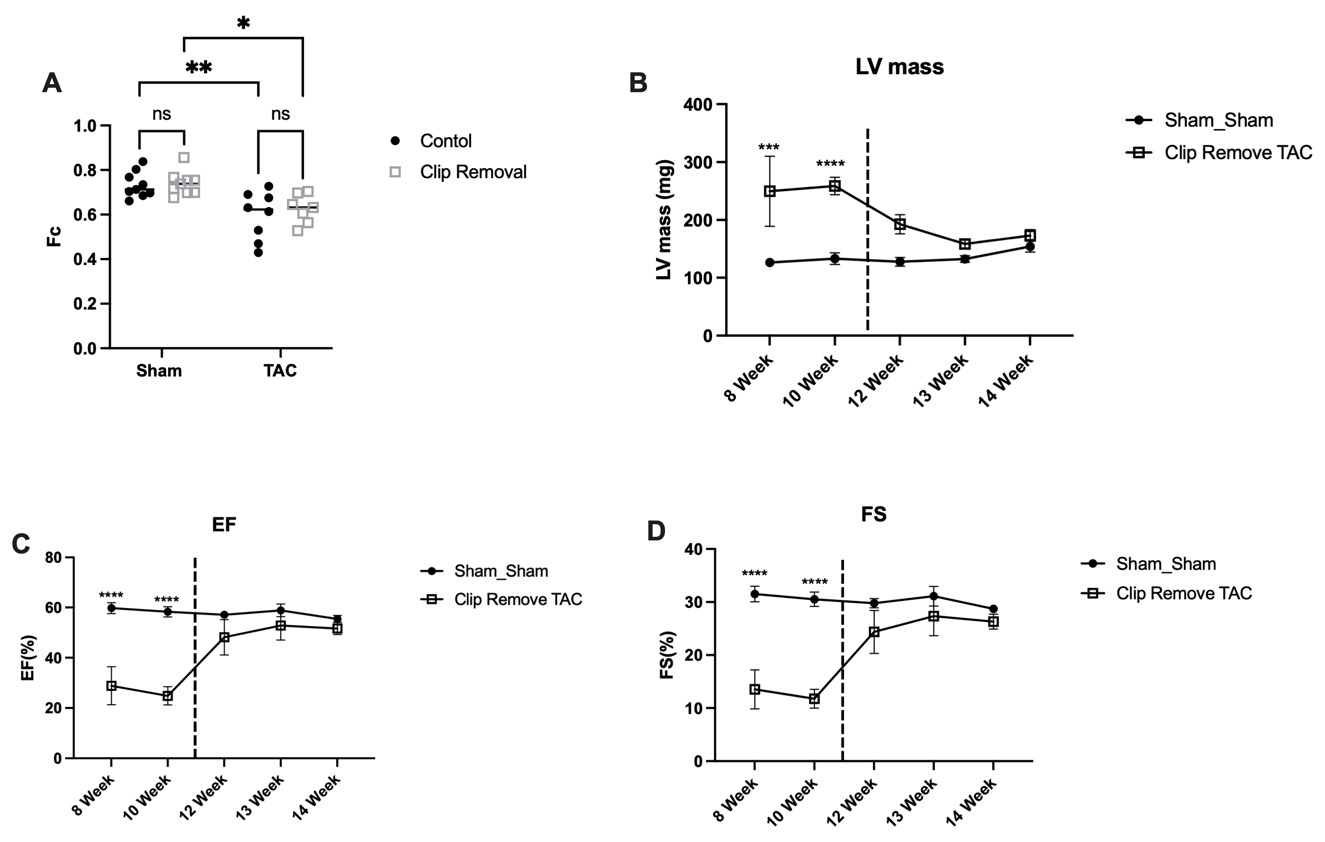
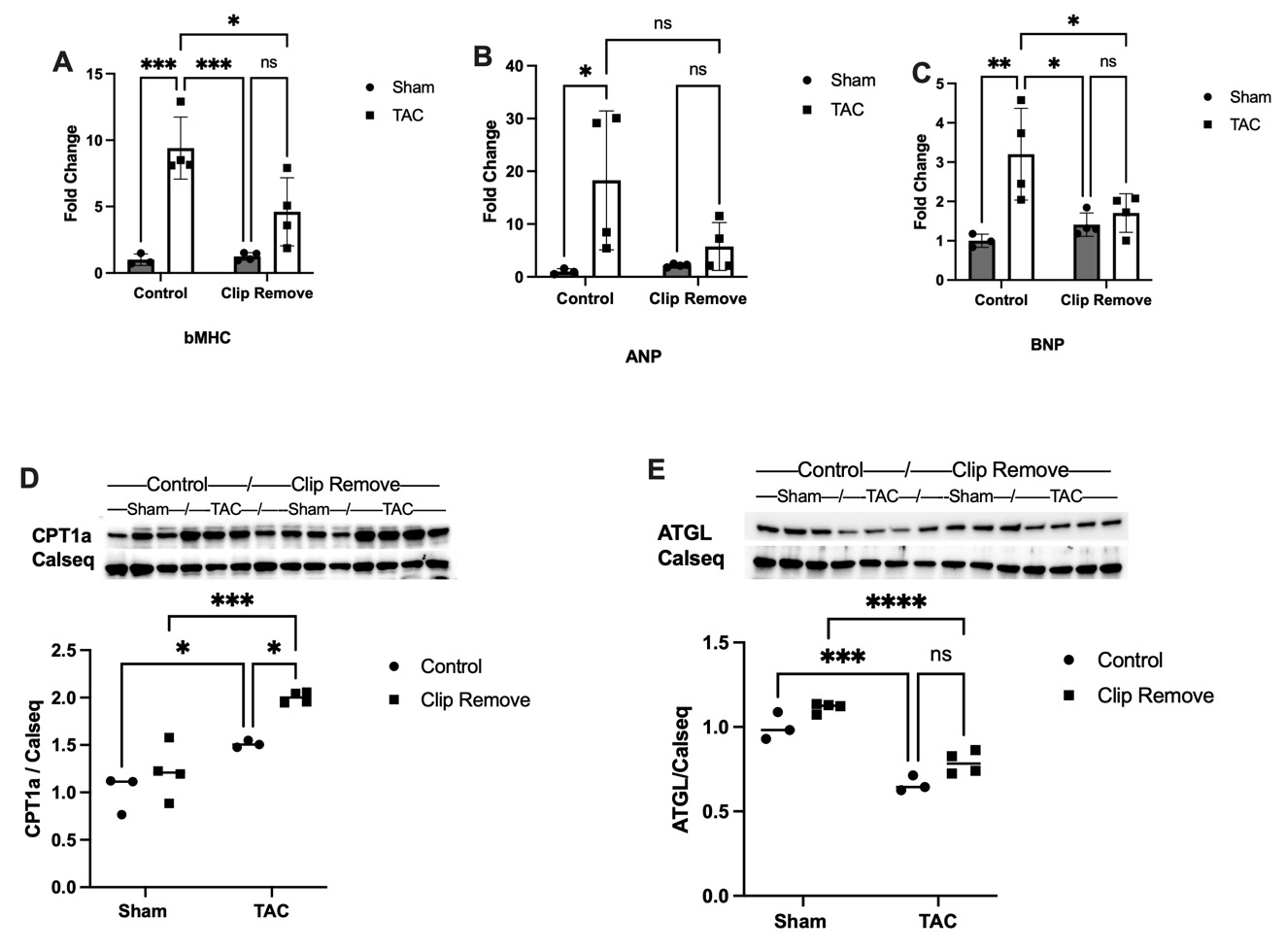
Results: TAC caused hypertrophy (LV mass +94%±14), and depressed EF (25±4% vs 60±8% in Sham) and fractional shortening (FS) (14±2% vs 32±1% in Sham) (Fig 1B,C,D). TAC induced characteristic FAO reductions by 18% (Fig 1A), and the stress induced isoform of carnitine palmitoyltransferase 1, Cpt1a increased 50% (Fig 2D). ATGL, the key lipase, fell 35% in TAC (Fig 2E), coinciding with characteristically reduced triglyceride (TG) by 43% (P<0.05).
At 3 weeks recovery, LV mass reverted, and EF (52 ± 3%) and FS (52 ± 3%) rebounded. Hypertrophic genes βMhc, Anp and Bnp (Fig 2A,B,C) in TAC-REM declined markedly. In contrast, FAO remained depressed with TAC-REM, 15.5% < Sham, with persistently elevated CPT1a, 70% > control. ATGL remained low, 22% of control, and low TG persisted in TAC-REM at 58% of control (P<0.05). Thus, pathologically remodeled LCFA metabolism despite recovery of EF and reduced LVH after removal of stress underscores a persistent constraint on LCFA metabolism.
Conclusions: This is the first evidence that cardiac metabolic remodeling persists even after pathological hypertrophy and dysfunction improve following alleviation of afterload stress. In a mouse model mimicking AVR for AS, heart function improved, but FAO remained impaired. CPT1a stayed elevated, and ATGL was suppressed, limiting LCFA metabolism. Findings reveal a metabolic restriction that lingers despite functional recovery, highlighting metabolic defects as a key target for complete recovery from pressure-overload cardiomyopathy.
Methods: Adult male C57BL/6J mice underwent Sham surgery, TAC (11wks), or TAC-REM (TAC removal at 11wks with 3 wks recovery). In vivo function was monitored via echocardiography. At endpoint, isolated hearts were perfused with a physiological mix of 13C enriched LCFA, 13C-palmitate, with glucose and lactate. In vitro 13C NMR provided fractional contribution (Fc) to oxidative metabolism. Markers of LVH and metabolic remodeling were assessed by qPCR and immunoblot.
Results: TAC caused hypertrophy (LV mass +94%±14), and depressed EF (25±4% vs 60±8% in Sham) and fractional shortening (FS) (14±2% vs 32±1% in Sham) (Fig 1B,C,D). TAC induced characteristic FAO reductions by 18% (Fig 1A), and the stress induced isoform of carnitine palmitoyltransferase 1, Cpt1a increased 50% (Fig 2D). ATGL, the key lipase, fell 35% in TAC (Fig 2E), coinciding with characteristically reduced triglyceride (TG) by 43% (P<0.05).
At 3 weeks recovery, LV mass reverted, and EF (52 ± 3%) and FS (52 ± 3%) rebounded. Hypertrophic genes βMhc, Anp and Bnp (Fig 2A,B,C) in TAC-REM declined markedly. In contrast, FAO remained depressed with TAC-REM, 15.5% < Sham, with persistently elevated CPT1a, 70% > control. ATGL remained low, 22% of control, and low TG persisted in TAC-REM at 58% of control (P<0.05). Thus, pathologically remodeled LCFA metabolism despite recovery of EF and reduced LVH after removal of stress underscores a persistent constraint on LCFA metabolism.
Conclusions: This is the first evidence that cardiac metabolic remodeling persists even after pathological hypertrophy and dysfunction improve following alleviation of afterload stress. In a mouse model mimicking AVR for AS, heart function improved, but FAO remained impaired. CPT1a stayed elevated, and ATGL was suppressed, limiting LCFA metabolism. Findings reveal a metabolic restriction that lingers despite functional recovery, highlighting metabolic defects as a key target for complete recovery from pressure-overload cardiomyopathy.
More abstracts on this topic:
Alleviating Aortic Valve Calcification By Blocking TNFα Receptors
Thent Zar Chi, Butcher Jonathan, Zhou Bin
Acute sleep deprivation induces cardiac remodeling via activation of AT1R/ERK/GSK-3β signalingLuo Tao, Liu Haiqiong