Final ID: MP368
Evaluating Aldosterone Synthase Inhibitors in Hypertension: A Meta-Analysis of Efficacy, Safety, and Subgroup Outcomes Across Novel Agents
Abstract Body (Do not enter title and authors here): Background:
Hypertension remains a leading risk factor for cardiovascular disease and mortality. Aldosterone, a critical mineralocorticoid hormone, regulates salt, water balance, and blood pressure. Aldosterone synthase inhibitor (ASI) are a novel class of drugs targeting this pathway to control blood pressure.
Hypothesis:
This study is focused on the efficacy and safety of ASIs in the treatment of hypertension, with subgroup analysis based on drug type and patient population.
Methods:
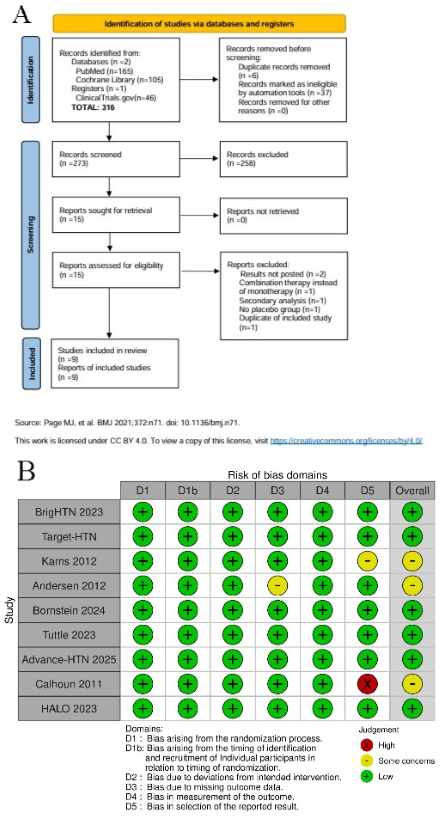
A comprehensive literature search was conducted using PubMed, Cochrane Library, and ClinicalTrials.gov for randomized controlled trials (RCT) published up to May 2025. Only RCTs comparing ASIs and placebo in adults (age > 18) with hypertension were included. Four different ASIs - lorundrostat, osilodrostat, baxdrostat, and vicadrostat- were evaluated across the included trials. Data analysis was performed on RevMan 5.4 using random-effects model with the Mantel-Haenszel statistical method to estimate the mean difference (MD) and 95 % confidence intervals between the drugs and placebo groups.
Results:
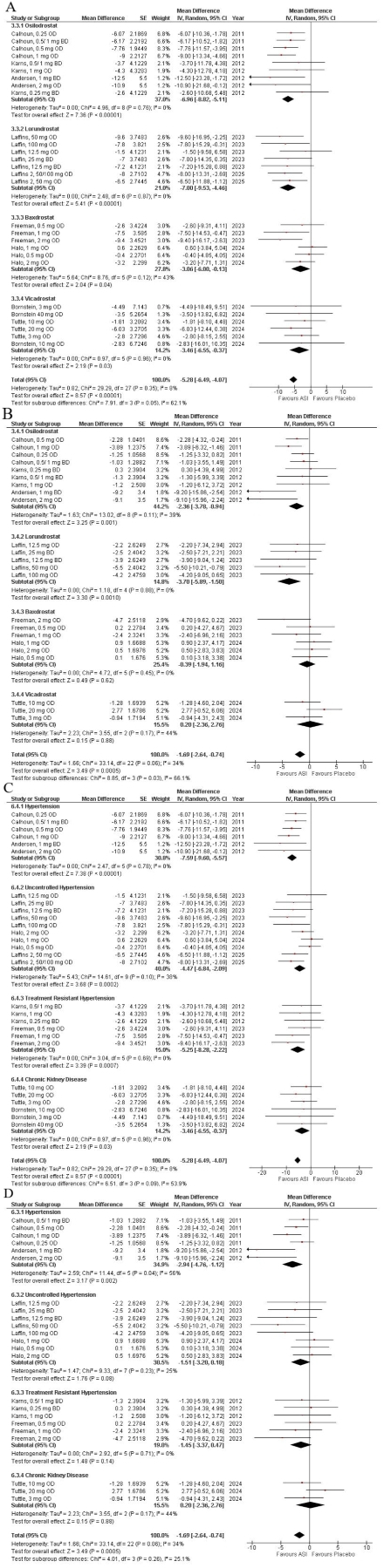
Nine RCTs were included with a total of 1774 patients with hypertension (354 lurondrostat, 500 osilodrostat, 391 baxdrostat, 260 vicadrostat and 469 placebo). Pooled analysis found that ASIs significantly reduced systolic (MD: -5.28, 95% CI: -6.49, -4.07: p<0.00001) and diastolic BP (MD: -1.69, 95% CI: –2.64, -0.74: p=0.0005) with efficacy varying by agent and population. Among different agents, lorundrostat showed the most pronounced reduction in SBP (MD: -7.00, 95% CI: -9.53, -4.46: p<00001) and DBP (MD: -3.70, 95% CI: -5.89, -1.50: p=0.0010) while Baxdrostat showed the least reduction in SBP(MD: -3.06, 95% CI: -6.00, -0.13: p=0.04) and DBP(MD: -0.39, 95% CI: -1.94, 1.16: p=0.62) respectively. Amongst the patient population, SBP reduction was the highest in patients with essential hypertension (MD: -7.59, 95% CI: -9.60, -5.57; p<0.00001) and lowest in CKD patients (MD: -3.46, 95% CI: -6.55, -0.37; p=0.0010). Overall, the ASIs were well tolerated with no serious (RR: 0.81, 95% CI: 0.44, 1.49: p=0.49) or any adverse events (RR: 1.01, 95% CI: 0.83,1.24: p=0.90).
Conclusion:
ASIs appear to be effective and well-tolerated antihypertensive agents, offering significant BP reduction across diverse patient groups, with lorundrostat demonstrating the strongest efficacy. These findings support further large-scale head-to-head trials to better define their role in hypertension management.
Hypertension remains a leading risk factor for cardiovascular disease and mortality. Aldosterone, a critical mineralocorticoid hormone, regulates salt, water balance, and blood pressure. Aldosterone synthase inhibitor (ASI) are a novel class of drugs targeting this pathway to control blood pressure.
Hypothesis:
This study is focused on the efficacy and safety of ASIs in the treatment of hypertension, with subgroup analysis based on drug type and patient population.
Methods:
A comprehensive literature search was conducted using PubMed, Cochrane Library, and ClinicalTrials.gov for randomized controlled trials (RCT) published up to May 2025. Only RCTs comparing ASIs and placebo in adults (age > 18) with hypertension were included. Four different ASIs - lorundrostat, osilodrostat, baxdrostat, and vicadrostat- were evaluated across the included trials. Data analysis was performed on RevMan 5.4 using random-effects model with the Mantel-Haenszel statistical method to estimate the mean difference (MD) and 95 % confidence intervals between the drugs and placebo groups.
Results:
Nine RCTs were included with a total of 1774 patients with hypertension (354 lurondrostat, 500 osilodrostat, 391 baxdrostat, 260 vicadrostat and 469 placebo). Pooled analysis found that ASIs significantly reduced systolic (MD: -5.28, 95% CI: -6.49, -4.07: p<0.00001) and diastolic BP (MD: -1.69, 95% CI: –2.64, -0.74: p=0.0005) with efficacy varying by agent and population. Among different agents, lorundrostat showed the most pronounced reduction in SBP (MD: -7.00, 95% CI: -9.53, -4.46: p<00001) and DBP (MD: -3.70, 95% CI: -5.89, -1.50: p=0.0010) while Baxdrostat showed the least reduction in SBP(MD: -3.06, 95% CI: -6.00, -0.13: p=0.04) and DBP(MD: -0.39, 95% CI: -1.94, 1.16: p=0.62) respectively. Amongst the patient population, SBP reduction was the highest in patients with essential hypertension (MD: -7.59, 95% CI: -9.60, -5.57; p<0.00001) and lowest in CKD patients (MD: -3.46, 95% CI: -6.55, -0.37; p=0.0010). Overall, the ASIs were well tolerated with no serious (RR: 0.81, 95% CI: 0.44, 1.49: p=0.49) or any adverse events (RR: 1.01, 95% CI: 0.83,1.24: p=0.90).
Conclusion:
ASIs appear to be effective and well-tolerated antihypertensive agents, offering significant BP reduction across diverse patient groups, with lorundrostat demonstrating the strongest efficacy. These findings support further large-scale head-to-head trials to better define their role in hypertension management.
More abstracts on this topic:
A RETRO-ENANTIOMER OF ANGIOTENSIN-(1-9) PREVENTS THE DEVELOPMENT OF HEART FAILURE WITH PRESERVED EJECTION FRACTION.
Ocaranza Maria Paz, Jimenez Veronica, Yanez Osvaldo, Jalil Jorge, Venegas Camilo, Candia Camila, Hermoso Marcela, Gabrielli Luigi, Morales Javier, Oyarzun Felipe, Torres Cristian, Lillo Pablo
A Multicenter Friedreich Ataxia Registry Identifies Posterior Wall Thickness as a Predictor of Major Adverse Cardiac EventsLin Kimberly, Johnson Jonathan, Mccormack Shana, Lynch David, Tate Barbara, Feng Yixuan, Huang Jing, Mercer-rosa Laura, Dedio Anna, Mcsweeney Kara, Fournier Anne, Yoon Grace, Payne Ronald, Cripe Linda, Patel Aarti, Niaz Talha