Final ID: Su3117
Clinical Effectiveness of Cerebral Embolic Protection (CEP) Devices During Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis
Abstract Body (Do not enter title and authors here): Introduction: Patients undergoing Transcatheter Aortic Valve Replacement/Implantation (TAVR/TAVI) are at risk of periprocedural cerebrovascular events. Cerebral Embolic Protection (CEP) devices have been developed to reduce the incidence of stroke and improve short-term outcomes in this population. This meta-analysis aimed to evaluate the safety and efficacy of CEP devices in patients undergoing TAVR/TAVI.
Methods: A comprehensive literature search was conducted using PubMed, Cochrane Library, and ClinicalTrials.gov to identify relevant randomized controlled trials using keywords like “Transcatheter aortic valve replacement” or “Transcatheter aortic valve implantation” and “Cerebral Embolic Protection device”. After screening and eligibility assessment, 6 articles were included from an initial pool of 157 records. Meta-analysis was performed using RevMan 5.4 software. Quality assessment was performed using the Cochrane Risk of Bias tool.
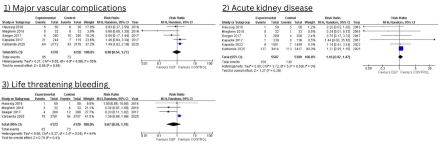
Results: Final analysis included 11,600 patients to investigate the relative effect of CEP devices versus no protection on different efficacy and safety outcomes in patients undergoing TAVR/TAVI. The pooled analysis demonstrated a non-significant reduction in the risk of stroke within 30 days in the CEP group compared to the control group (RR=0.81; 95% CI 0.61 to 1.07; p=0.14, I2 =14%). The use of CEP was not associated with a significant difference in all-cause mortality (RR=0.89; 95% CI 0.50 to 1.58; p=0.68, I2=18%), disabling stroke (RR=0.53; 95% CI 0.26 to 1.07; p=0.08, I2=44%) and non-disabling stroke (RR=0.92; 95% CI 0.61 to 1.39; p=0.69, I2=0%) within 30 days. Safety outcomes showed no significant differences between CEP and control groups in terms of major vascular complications (RR=0.98; 95% CI 054 to 1.77; p=0.95, I2 =50%), major or life-threatening bleeding (RR=0.67; 95% CI 0.26 to 1.78; p=0.43, I2 =64%), and acute kidney injury (RR=1.16; 95% CI 0.92 to 1.47; p=0.20, I2=0%).
Discussion: CEP devices may reduce the risk of disabling stroke in patients undergoing TAVR/TAVI; however, the reduction did not reach statistical significance. Additionally, there was no significant benefit observed in all-cause mortality or other efficacy and safety outcomes. These findings highlight the need for larger, high-quality trials to better clarify the clinical value of CEP devices in this setting.
Methods: A comprehensive literature search was conducted using PubMed, Cochrane Library, and ClinicalTrials.gov to identify relevant randomized controlled trials using keywords like “Transcatheter aortic valve replacement” or “Transcatheter aortic valve implantation” and “Cerebral Embolic Protection device”. After screening and eligibility assessment, 6 articles were included from an initial pool of 157 records. Meta-analysis was performed using RevMan 5.4 software. Quality assessment was performed using the Cochrane Risk of Bias tool.
Results: Final analysis included 11,600 patients to investigate the relative effect of CEP devices versus no protection on different efficacy and safety outcomes in patients undergoing TAVR/TAVI. The pooled analysis demonstrated a non-significant reduction in the risk of stroke within 30 days in the CEP group compared to the control group (RR=0.81; 95% CI 0.61 to 1.07; p=0.14, I2 =14%). The use of CEP was not associated with a significant difference in all-cause mortality (RR=0.89; 95% CI 0.50 to 1.58; p=0.68, I2=18%), disabling stroke (RR=0.53; 95% CI 0.26 to 1.07; p=0.08, I2=44%) and non-disabling stroke (RR=0.92; 95% CI 0.61 to 1.39; p=0.69, I2=0%) within 30 days. Safety outcomes showed no significant differences between CEP and control groups in terms of major vascular complications (RR=0.98; 95% CI 054 to 1.77; p=0.95, I2 =50%), major or life-threatening bleeding (RR=0.67; 95% CI 0.26 to 1.78; p=0.43, I2 =64%), and acute kidney injury (RR=1.16; 95% CI 0.92 to 1.47; p=0.20, I2=0%).
Discussion: CEP devices may reduce the risk of disabling stroke in patients undergoing TAVR/TAVI; however, the reduction did not reach statistical significance. Additionally, there was no significant benefit observed in all-cause mortality or other efficacy and safety outcomes. These findings highlight the need for larger, high-quality trials to better clarify the clinical value of CEP devices in this setting.
More abstracts on this topic:
A Diagnostic Pitfall: Subclavian Stenosis Mimicking Severe Aortic Stenosis on Echocardiography"
Ezaldin Shady, Abdelsalam Mahmoud, Elsayed Omar, Lee Marciano
A Meta-Analysis Comparing Same-Day Discharge to Later-Day Discharge in Transcatheter Aortic Valve ReplacementJain Hritvik, Passey Siddhant, Jain Jyoti, Goyal Aman, Wasir Amanpreet, Ahmed Mushood, Patel Nandan, Yadav Ashish, Shah Janhvi, Mehta Aryan