Final ID: MP2256
Vutrisiran Healthcare Resource Utilization, Costs, Discontinuation, and Mortality: a Retrospective Database Analysis
Abstract Body (Do not enter title and authors here): Introduction: Vutrisiran is approved for hereditary transthyretin amyloid polyneuropathy (06/2022) and transthyretin amyloid cardiomyopathy (ATTR-CM; 03/2025). Real-world data on vutrisiran healthcare resource utilization (HCRU), costs, discontinuation, and mortality are limited.
Objective: To report real-world vutrisiran HCRU, costs, discontinuation, and mortality in the US.
Methods: Retrospective analysis of Optum® Clinformatics® Data Mart patients (pt) initiating vutrisiran from 2022 to 2023 (before ATTR-CM approval) with ≥1 vutrisiran claim and ≥1 y continuous enrollment after initiation. Pts were followed until death, enrollment end, or last date of available data. Hospitalizations, emergency department (ED) visits, and associated costs were assessed. Discontinuation was defined as a ≥180-d gap (90-d supply + 90-d grace period) between treatments. Analyses replicated in Komodo Healthcare Map® served as a comparator.
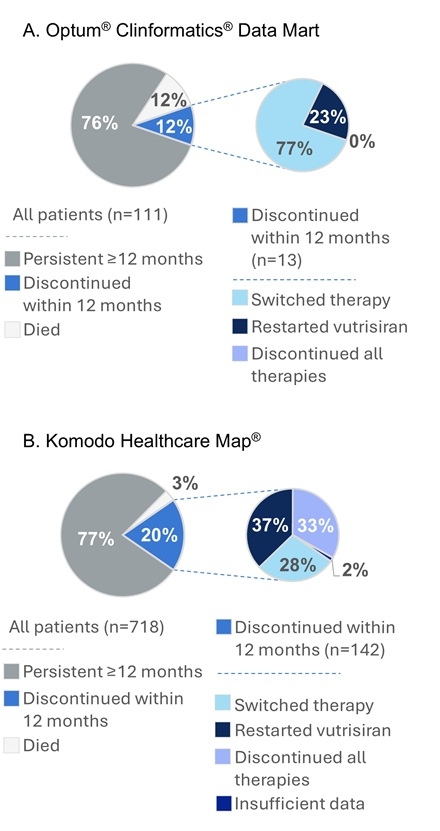
Results: In the Optum data set (n=111), mean (SD) age was 70.9 y (9.9), and the majority were men (62%); 41% were Black and 27% were White, and 30% were race unknown. Mean (SD) follow-up was 1.5 y (0.6). After vutrisiran initiation, 41 (37%) pts had hospitalizations with a mean (SD) of 16.0 d (21.4) annually at a cost of $80,536 ($83,907) per pt. Also, 55 (50%) pts had ED visits not leading to hospitalization, with a mean of 2.4 visits and mean (SD) ED costs of $6339 ($7484) annually per pt. Within 12 mo of starting vutrisiran, 13 (12%) pts died and another 13 (12%) pts discontinued therapy; of discontinued pts, 3 (23%) subsequently restarted vutrisiran and 10 (77%) switched ATTR therapy (Figure 1A).
In Komodo (n=718), mean (SD) age was 71.0 y (11.0); pts were 61% men, 43% were Black, and 39% were White (unknown race, 4%). Within 12 mo, 22 (3%) pts died and another 142 (20%) discontinued vutrisiran. Of those who discontinued, 53 (37%) later restarted vutrisiran, 40 (28%) switched ATTR therapy, and 47 (33%) discontinued all treatments (Figure 1B).
Conclusions: One-third to half of pts presented to the hospital within 1.5 y of vutrisiran treatment (before ATTR-CM approval); within 12 mo, roughly 1 in 4 died or discontinued therapy for ≥90 d past the 90-d administration schedule, with many switching to other ATTR treatments. This suggests that vutrisiran-treated pts continue to experience significant HCRU and costs related to disease progression despite treatment. A need remains for new therapies to further reduce burden and costs.
Objective: To report real-world vutrisiran HCRU, costs, discontinuation, and mortality in the US.
Methods: Retrospective analysis of Optum® Clinformatics® Data Mart patients (pt) initiating vutrisiran from 2022 to 2023 (before ATTR-CM approval) with ≥1 vutrisiran claim and ≥1 y continuous enrollment after initiation. Pts were followed until death, enrollment end, or last date of available data. Hospitalizations, emergency department (ED) visits, and associated costs were assessed. Discontinuation was defined as a ≥180-d gap (90-d supply + 90-d grace period) between treatments. Analyses replicated in Komodo Healthcare Map® served as a comparator.
Results: In the Optum data set (n=111), mean (SD) age was 70.9 y (9.9), and the majority were men (62%); 41% were Black and 27% were White, and 30% were race unknown. Mean (SD) follow-up was 1.5 y (0.6). After vutrisiran initiation, 41 (37%) pts had hospitalizations with a mean (SD) of 16.0 d (21.4) annually at a cost of $80,536 ($83,907) per pt. Also, 55 (50%) pts had ED visits not leading to hospitalization, with a mean of 2.4 visits and mean (SD) ED costs of $6339 ($7484) annually per pt. Within 12 mo of starting vutrisiran, 13 (12%) pts died and another 13 (12%) pts discontinued therapy; of discontinued pts, 3 (23%) subsequently restarted vutrisiran and 10 (77%) switched ATTR therapy (Figure 1A).
In Komodo (n=718), mean (SD) age was 71.0 y (11.0); pts were 61% men, 43% were Black, and 39% were White (unknown race, 4%). Within 12 mo, 22 (3%) pts died and another 142 (20%) discontinued vutrisiran. Of those who discontinued, 53 (37%) later restarted vutrisiran, 40 (28%) switched ATTR therapy, and 47 (33%) discontinued all treatments (Figure 1B).
Conclusions: One-third to half of pts presented to the hospital within 1.5 y of vutrisiran treatment (before ATTR-CM approval); within 12 mo, roughly 1 in 4 died or discontinued therapy for ≥90 d past the 90-d administration schedule, with many switching to other ATTR treatments. This suggests that vutrisiran-treated pts continue to experience significant HCRU and costs related to disease progression despite treatment. A need remains for new therapies to further reduce burden and costs.
More abstracts on this topic:
Causative Organism Predicts Diagnostic Utility of TTE in Bacteremia-Associated Endocarditis
Obaed Nadia, Nevin Andrew, Foy Andrew
A Contemporary Machine Learning-Based Risk Stratification for Mortality and Hospitalization in Heart Failure with Preserved Ejection Fraction Using Multimodal Real-World DataFudim Marat, Weerts Jerremy, Patel Manesh, Balu Suresh, Hintze Bradley, Torres Francisco, Micsinai Balan Mariann, Rigolli Marzia, Kessler Paul, Touzot Maxime, Lund Lars, Van Empel Vanessa, Pradhan Aruna, Butler Javed, Zehnder Tobias, Sauty Benoit, Esposito Christian, Balazard Félix, Mayer Imke, Hallal Mohammad, Loiseau Nicolas