Final ID: MP486
Demographic Disparities in Tafamidis Treatment and Clinical Outcomes Across the United States
Abstract Body (Do not enter title and authors here): Introduction: Transthyretin amyloid cardiomyopathy (ATTR-CM) is a progressive, often fatal disease. A better understanding of demographic disparities in diagnosis and treatment is crucial to optimize care and improve outcomes across diverse patient (pt) populations.
Objectives: To evaluate potential differences in initiation of tafamidis, the only approved ATTR-CM therapy at the time of the study, and subsequent clinical outcomes by gender and race. We hypothesized that significant demographic differences exist in treatment patterns and clinical outcomes.
Methods: We conducted a retrospective cohort analysis using the US Komodo Healthcare Map® (01/2016-06/2024). Pts with amyloidosis ICD-10-CM diagnosis codes were identified and followed from diagnosis to tafamidis initiation and cardiovascular-related hospitalization (CVH) or death. Cumulative incidence of treatment initiation and survival probabilities were stratified by gender and race.
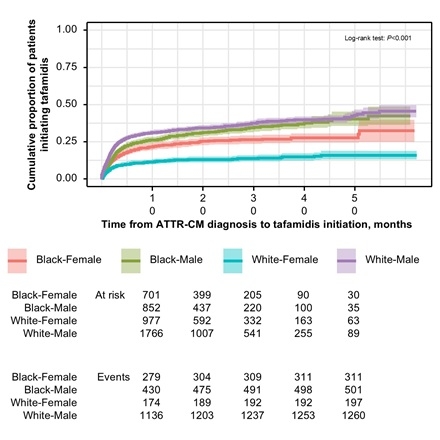
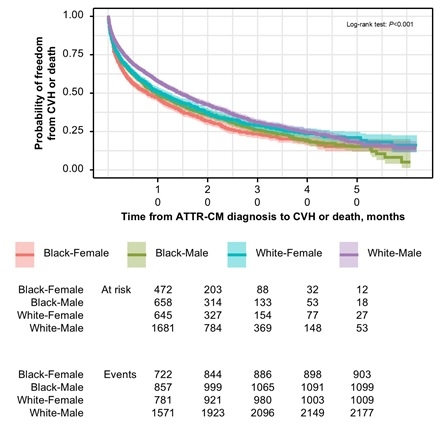
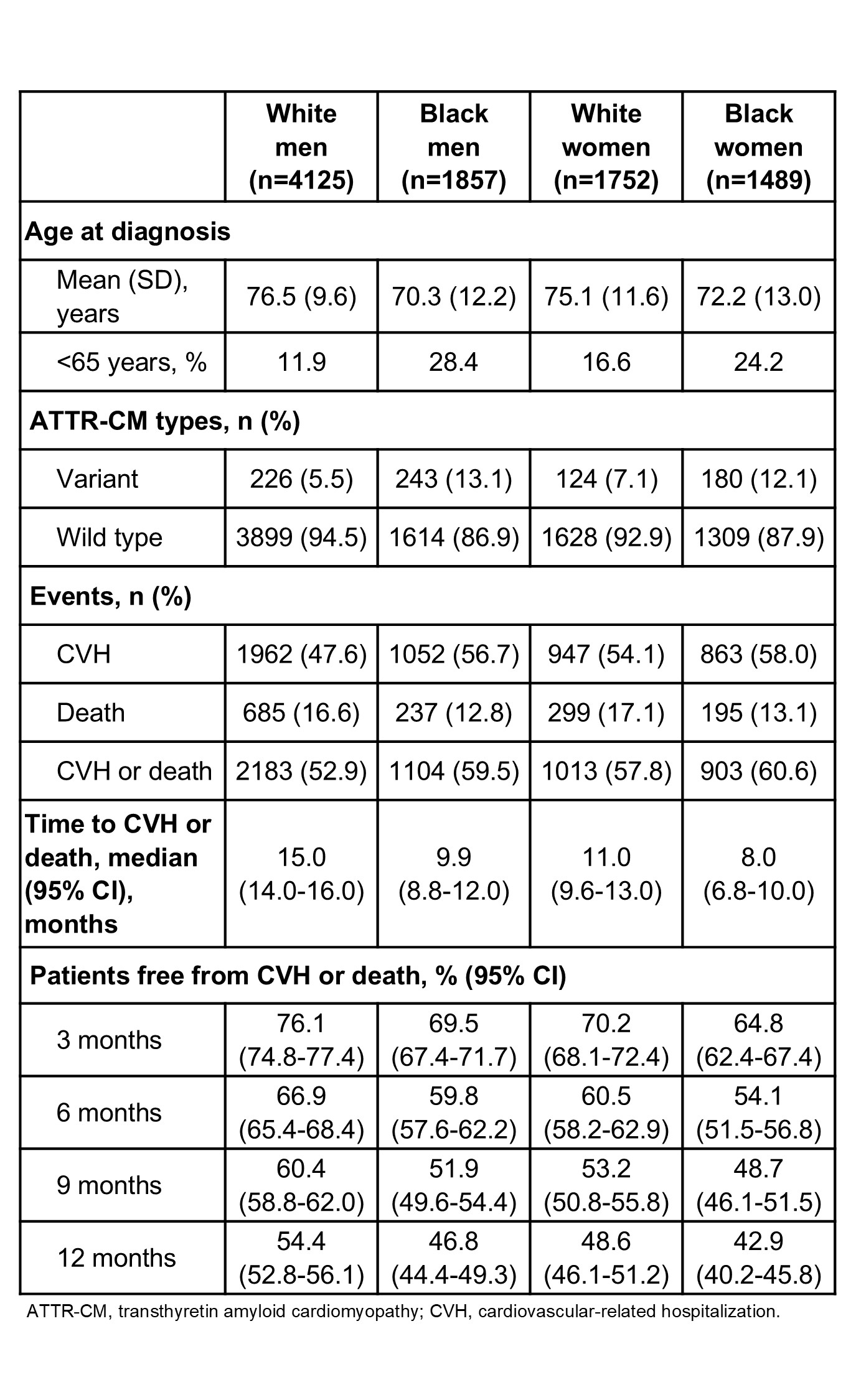
Results: We identified 11,311 pts with ATTR-CM (63.9% men [mean age, 73.5 y] and 36.1% women [mean age, 72.4 y]). After diagnosis, women had a significantly lower cumulative incidence of tafamidis initiation compared to men at all time points (P<0.001). At 3 mo after diagnosis, the cumulative incidence was 9.9% among women versus 19.9% among men; by 12 mo, this gap persisted (14.5% vs 28.5%). Race further compounded these disparities (P<0.001; Figure 1). At 12 mo, White men had the highest cumulative incidence of initiation (31.0%), followed by Black men (26.7%), Black women (22.0%), and White women (11.4%). CVH or death occurred in 57.7% of women versus 53.6% of men. Event-free survival at 12 mo was lowest in Black women (42.9%) versus Black men (46.8%), White women (48.6%), and White men (54.4%) (P<0.001; Figure 2). Black women experienced the shortest median (95% CI) time to CVH or death (8.0 mo [6.8-10.0]), followed by Black men (9.9 mo [8.8-12.0]), White women (11.0 mo [9.6-13.0]), and White men (15.0 mo [14.0-16.0]; Table).
Conclusion: This large-scale analysis of a US cohort suggests existing gender and racial disparities in tafamidis treatment initiation and outcomes in ATTR-CM. White women had the lowest rates of tafamidis initiation, while Black women had the worst clinical outcomes, highlighting a compounded disparity in treatment and survival by gender and race. These findings underscore the urgent need to address demographic-based disparities and ensure equitable care for all pts with ATTR-CM.
Objectives: To evaluate potential differences in initiation of tafamidis, the only approved ATTR-CM therapy at the time of the study, and subsequent clinical outcomes by gender and race. We hypothesized that significant demographic differences exist in treatment patterns and clinical outcomes.
Methods: We conducted a retrospective cohort analysis using the US Komodo Healthcare Map® (01/2016-06/2024). Pts with amyloidosis ICD-10-CM diagnosis codes were identified and followed from diagnosis to tafamidis initiation and cardiovascular-related hospitalization (CVH) or death. Cumulative incidence of treatment initiation and survival probabilities were stratified by gender and race.
Results: We identified 11,311 pts with ATTR-CM (63.9% men [mean age, 73.5 y] and 36.1% women [mean age, 72.4 y]). After diagnosis, women had a significantly lower cumulative incidence of tafamidis initiation compared to men at all time points (P<0.001). At 3 mo after diagnosis, the cumulative incidence was 9.9% among women versus 19.9% among men; by 12 mo, this gap persisted (14.5% vs 28.5%). Race further compounded these disparities (P<0.001; Figure 1). At 12 mo, White men had the highest cumulative incidence of initiation (31.0%), followed by Black men (26.7%), Black women (22.0%), and White women (11.4%). CVH or death occurred in 57.7% of women versus 53.6% of men. Event-free survival at 12 mo was lowest in Black women (42.9%) versus Black men (46.8%), White women (48.6%), and White men (54.4%) (P<0.001; Figure 2). Black women experienced the shortest median (95% CI) time to CVH or death (8.0 mo [6.8-10.0]), followed by Black men (9.9 mo [8.8-12.0]), White women (11.0 mo [9.6-13.0]), and White men (15.0 mo [14.0-16.0]; Table).
Conclusion: This large-scale analysis of a US cohort suggests existing gender and racial disparities in tafamidis treatment initiation and outcomes in ATTR-CM. White women had the lowest rates of tafamidis initiation, while Black women had the worst clinical outcomes, highlighting a compounded disparity in treatment and survival by gender and race. These findings underscore the urgent need to address demographic-based disparities and ensure equitable care for all pts with ATTR-CM.
More abstracts on this topic:
Association Between Perceived Discrimination and Depression/Anxiety Symptoms
Tang Zhengxin, Lv Nan, Ma Jun
A Predictive Tool and Diagnostic Screening Algorithm for the Identification of Transthyretin Amyloid Cardiomyopathy in High-Risk Patient PopulationsChai Jocelyn, Sathananthan Janarthanan, Fine Nowell, Davis Margot, Starovoytov Andrew, Campbell Christine, Hawkins Nathaniel, Virani Sean, Luong Michael, Straatman Lynn, Kiess Marla, Worsley Daniel