Final ID: MP717
Decoding Endocardial Fibroelastosis with Differentially Regulated miR-100-5p and miR-503-5p
Abstract Body (Do not enter title and authors here): Background: Endocardial fibroelastosis is a unique form of subendocardial fibrosis leading to diastolic dysfunction and heart failure. The root cause of EFE is the fibrogenic transition of endocardial endothelial cells (EECs) to mesenchymal cells known as endothelial-to-mesenchymal transition (EndMT). MicroRNAs (miRNAs) have already been identified as crucial epigenetic regulators of epithelial-to-mesenchymal transition in tumor growth and cancer, but little is known about their role in EndMT-driven cardiac fibrosis.
Hypothesis: miRNAs are uniquely expressed in EFE, and their downstream targets modulate EndMT.
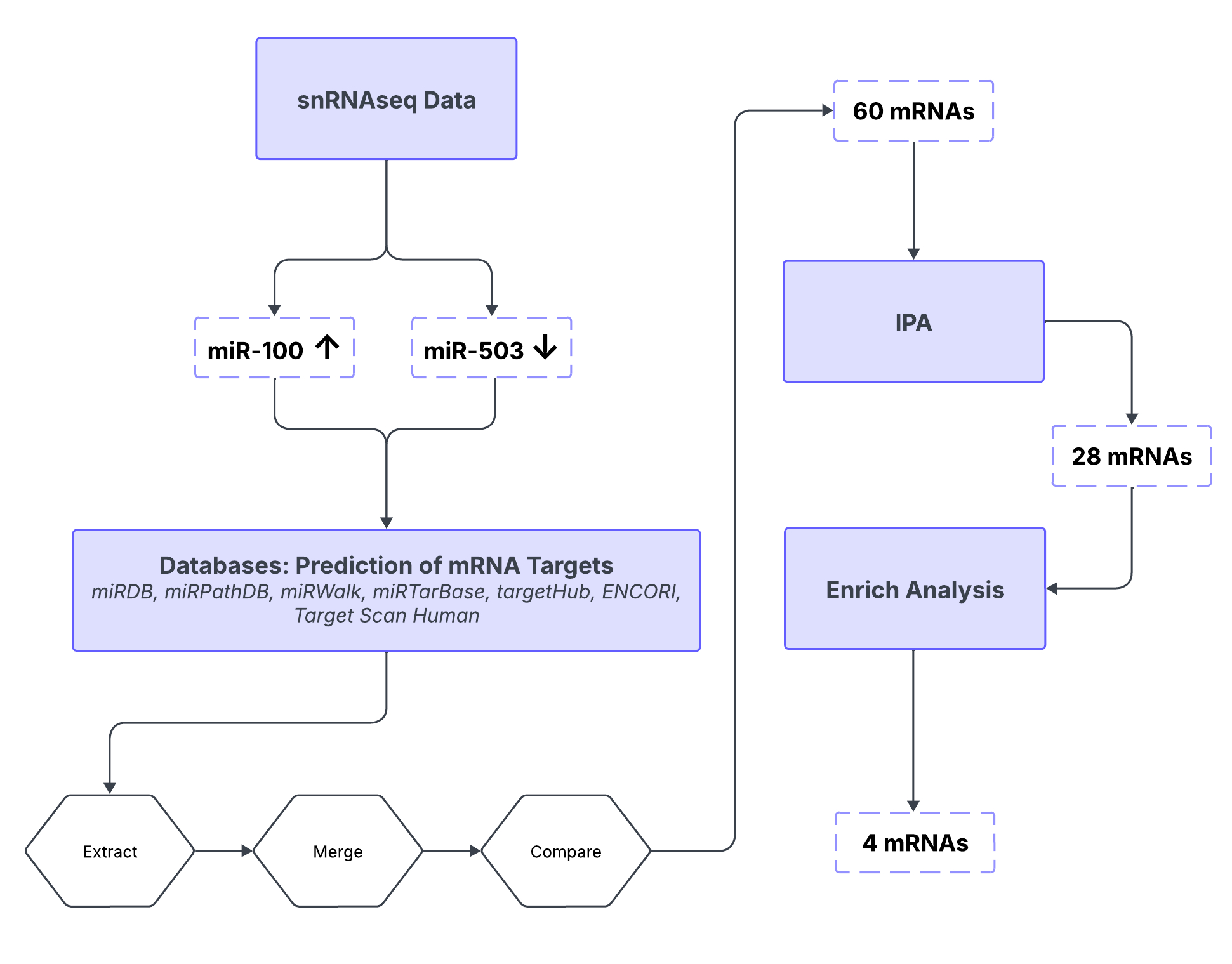
Methods: Expression levels of miRNAs were identified in snRNAseq data comparing EFE with age-matched healthy controls. Differentially regulated miRNAs were further reevaluated by qRT-PCR. In silico analyses were performed to predict mRNA targets of dysregulated specific miRNAs in EECs using open-access databases. Computational modeling filtered mRNA targets associated with the regulation of EndMT using Python, EnrichR, and Ingenuity Pathway Analysis (IPA), with qRT-PCR confirmation.
Results: Endocardial-derived clusters in snRNAseq data indicated two differentially regulated miRNAs: miR-100 upregulation and miR-503 downregulation. qRT-PCR confirmed the upregulation of miR-100-5p and downregulation of miR-503-5p in EFE tissue compared to healthy controls. In silico analysis predicted the involvement of both dysregulated miRNAs in EndMT by regulating downstream targets associated with the TGF-ß pathway, i.e., SKI and ZBTB7A for miR-100-5p, and SMAD7 for miR-503-5p. All are involved in the negative regulation of the TGF-ß pathway and are also targeting CYP26B1, a key regulator in early heart valve development. According to the in silico analysis, CYP26B1 expression levels were unaffected by the two miRNAs, as we confirmed in EFE compared to healthy tissue and cells by qRT-PCR. Additionally, snRNAseq data showed the downregulation of SKI, ZBTB7A, and the upregulation of SMAD7 in EEC-derived clusters compared to the controls. All experiments were conducted in biological triplicate. Data were analyzed using an unpaired t-test.
Conclusions: For the first time, we identified the dysregulation of two miRNAs, miR-100-5p and miR-503-5p, in EFE and their potential role in regulating EndMT. These findings highlight their possible use as ratio-based biomarkers and novel therapeutic targets in patients with EFE.
Hypothesis: miRNAs are uniquely expressed in EFE, and their downstream targets modulate EndMT.
Methods: Expression levels of miRNAs were identified in snRNAseq data comparing EFE with age-matched healthy controls. Differentially regulated miRNAs were further reevaluated by qRT-PCR. In silico analyses were performed to predict mRNA targets of dysregulated specific miRNAs in EECs using open-access databases. Computational modeling filtered mRNA targets associated with the regulation of EndMT using Python, EnrichR, and Ingenuity Pathway Analysis (IPA), with qRT-PCR confirmation.
Results: Endocardial-derived clusters in snRNAseq data indicated two differentially regulated miRNAs: miR-100 upregulation and miR-503 downregulation. qRT-PCR confirmed the upregulation of miR-100-5p and downregulation of miR-503-5p in EFE tissue compared to healthy controls. In silico analysis predicted the involvement of both dysregulated miRNAs in EndMT by regulating downstream targets associated with the TGF-ß pathway, i.e., SKI and ZBTB7A for miR-100-5p, and SMAD7 for miR-503-5p. All are involved in the negative regulation of the TGF-ß pathway and are also targeting CYP26B1, a key regulator in early heart valve development. According to the in silico analysis, CYP26B1 expression levels were unaffected by the two miRNAs, as we confirmed in EFE compared to healthy tissue and cells by qRT-PCR. Additionally, snRNAseq data showed the downregulation of SKI, ZBTB7A, and the upregulation of SMAD7 in EEC-derived clusters compared to the controls. All experiments were conducted in biological triplicate. Data were analyzed using an unpaired t-test.
Conclusions: For the first time, we identified the dysregulation of two miRNAs, miR-100-5p and miR-503-5p, in EFE and their potential role in regulating EndMT. These findings highlight their possible use as ratio-based biomarkers and novel therapeutic targets in patients with EFE.
More abstracts on this topic:
Aggressive Lipid Lowering Differentially Impacts the Vascular Endothelium in Diabetic vs Healthy Individuals. Findings from the American Heart Association Cardiometabolic Health Strategically Focused Research Network
Garshick Michael, Drenkova Kamelia, Schlamp Maria Florencia, Giannarelli Chiara, Fisher Edward, Goldberg Ira, Berger Jeffrey, Boothman Isabelle, Barrett Tessa, Jindal Manila, Newman Jonathan, Fadzan Maja, Bredefeld Cindy, Levy Natalie, Akinlonu Adedoyin
Impaired myocardial proliferation in cardioids derived from patients with hypoplastic left heart syndromeYu Yang, Wang Cankun, Ye Shiqiao, Texter Karen, Garg Vidu, Ma Qin, Zhao Mingtao