Final ID: MP723
Fibro-proliferative Remodeling of the Endocardium in Pulmonary Vein Stenosis
Abstract Body (Do not enter title and authors here): Background: Pathologically elevated shear stress and cellular proliferation are known to contribute to the luminal obstruction in pulmonary vein stenosis (PVS). The initial stenosis site is typically the veno-atrial junction, which is lined by endocardium. As we have shown previously, the endocardium is prone to fibroelastic disease through fibrogenic activation of endocardial endothelial cells (EECs) in response to elevated shear stress. However, the cellular pathomechanism in PVS is unkown.
Hypothesis: In response to altered wall shear stress, EECs undergo a multistep fibrogenic transition, including a pericyte-like intermediate stage, which identifies targets for selective therapy.
Methods: We obtained clinical data and PVS tissue (n=9) from cardiac surgery. Wall shear stress was calculated from preoperative CT images. We assessed tissue composition through light-microscopy and fibrogenic activation and regulation of EECs with immunohistochemical staining and flow cytometry on EECs for endothelial (CD31), early intermediate (3G5), late mesenchymal stage (vimentin), and TGF-β pathway activation through nuclear phospho-Smad2/3 and Slug/Snail markers.
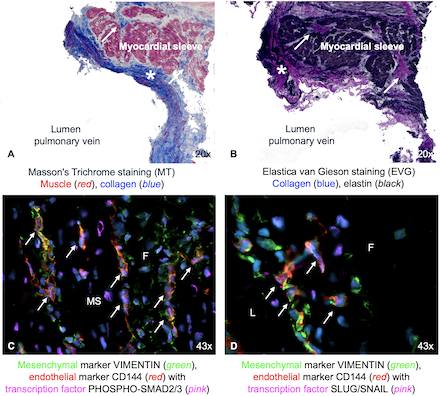
Results: Preoperatively, veins had a median gradient of 18mmHg (9-32) and pathological shear stress of 120 dyn/cm2 (42-155). Tissue proliferation was identified as fibroelastic remodeling (Fig1). Areas showed double positivity for endothelial and mesenchymal markers, with nuclear co-localization of transcription factors indicating active TGFβ-driven fibrogenic activation (Fig1). PVS tissue showed a highly fibrotic composition: 16±12% EECs, 54±19% mesenchymal, and 16±8% pericyte-like cells. Notably, 37±7% of EECs co-expressed 3G5, indicating endocardial lineage of pericyte-like (3G5+) intermediates. Among these 3G5+ cells, 80±12% were also vimentin-positive, reflecting acquisition of a fibrotic phenotype. PVS-EECs compared to healthy EECs exhibited a significantly higher proportion of cells across multiple intermediate transitioning stages and in late fibrogenic transition (Fig2).
Conclusion: Progressive intraluminal obstruction in PVS occurs as a result of localized fibrogenic activation of EECs in response to high shear stress. We present, for the first time, the multistage phenotypical transition of EECs in human PVS through a pericyte-like intermediary stage into fibroblasts. These intermediate stages now serve as therapeutic targets to identify optimized treatments for patients at various stages of disease.
Hypothesis: In response to altered wall shear stress, EECs undergo a multistep fibrogenic transition, including a pericyte-like intermediate stage, which identifies targets for selective therapy.
Methods: We obtained clinical data and PVS tissue (n=9) from cardiac surgery. Wall shear stress was calculated from preoperative CT images. We assessed tissue composition through light-microscopy and fibrogenic activation and regulation of EECs with immunohistochemical staining and flow cytometry on EECs for endothelial (CD31), early intermediate (3G5), late mesenchymal stage (vimentin), and TGF-β pathway activation through nuclear phospho-Smad2/3 and Slug/Snail markers.
Results: Preoperatively, veins had a median gradient of 18mmHg (9-32) and pathological shear stress of 120 dyn/cm2 (42-155). Tissue proliferation was identified as fibroelastic remodeling (Fig1). Areas showed double positivity for endothelial and mesenchymal markers, with nuclear co-localization of transcription factors indicating active TGFβ-driven fibrogenic activation (Fig1). PVS tissue showed a highly fibrotic composition: 16±12% EECs, 54±19% mesenchymal, and 16±8% pericyte-like cells. Notably, 37±7% of EECs co-expressed 3G5, indicating endocardial lineage of pericyte-like (3G5+) intermediates. Among these 3G5+ cells, 80±12% were also vimentin-positive, reflecting acquisition of a fibrotic phenotype. PVS-EECs compared to healthy EECs exhibited a significantly higher proportion of cells across multiple intermediate transitioning stages and in late fibrogenic transition (Fig2).
Conclusion: Progressive intraluminal obstruction in PVS occurs as a result of localized fibrogenic activation of EECs in response to high shear stress. We present, for the first time, the multistage phenotypical transition of EECs in human PVS through a pericyte-like intermediary stage into fibroblasts. These intermediate stages now serve as therapeutic targets to identify optimized treatments for patients at various stages of disease.
More abstracts on this topic:
β1 integrins regulate cellular behavior and cardiomyocyte organization during ventricular wall formation
Miao Lianjie, Schwartz Robert, R Burns Alan, Kumar Ashok, Dipersio C. Michael, Wu Mingfu, Lu Yangyang, Nusrat Anika, Zhao Luqi, Castillo Micah, Xiao Yongqi, Guo Hongyan, Liu Yu, Gunaratne Preethi
Aberrant Regulation of endMT in Turner Syndrome: Implications for the Pathogenesis of Congenital Cardiovascular DiseaseGarcia Huitron Eric Ivan, Zhang Xiaoying, Babcock Lance, Grande-allen Kathryn, Prakash Siddharth