Final ID: MP725
Imatinib Mesylate and Sirolimus Inhibit Fibrogenic Activation of Endocardial Endothelial Cells
Abstract Body (Do not enter title and authors here): Background: Clinically, Imatinib mesylate, a tyrosine kinase pathway inhibitor, and sirolimus, an mTOR inhibitor, are used as antiproliferative agents for the treatment of fibroelastic congenital heart disease. Both drugs also have anti-fibrotic properties by modulating the non-canonical TGFβ-1 pathway. We have previously demonstrated that fibroelastic disease is driven by fibrogenic activation of endocardial endothelial cells (EECs), primarily mediated through the TGFβ-1 signaling pathway.
Hypothesis: Imatinib mesylate and sirolimus safely and effectively suppress fibrogenic activation of EECs.
Methods: EECs were isolated from fibroelastic cardiac tissue obtained during routine surgeries and grown in culture. Cells were treated with clinically relevant concentrations of imatinib mesylate (0.1, 0.5, 1μM), sirolimus (5, 10, and 20nM), vehicle control (0.1% DMSO), or left untreated (native control). The medium concentrations within the therapeutic range were used for subsequent experiments. Cytotoxicity was assessed using Annexin V/PI staining by flow cytometry at 24 hours, and cell proliferation by MTT assay after 48 hours. Anti-fibrogenic effects were evaluated following 24 hours of TGFβ-1 stimulation (to induce fibrogenic activation) using immunocytochemistry for co-expression of endothelial marker CD144 and mesenchymal marker α-SMA, and mean α-SMA expression. All experiments were performed in biological triplicate. Flow cytometry analysis of CD31 and fibroblast-specific markers was performed to confirm anti-fibrogenic effects.
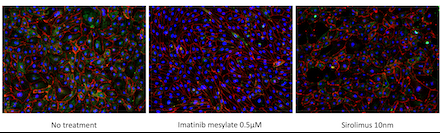
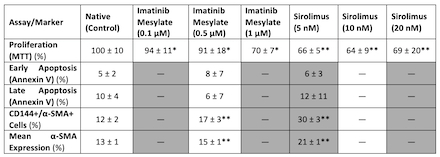
Results: Imatinib and Sirolimus significantly reduced proliferation compared to native and vehicle controls (*p<0.0002, **p<0.0001) (Table 1). Annexin V/PI staining revealed no significant differences in early or late apoptotic cell populations among treated groups and controls (p=0.83 and p=0.75, respectively). Treatment with both Imatinib and Sirolimus resulted in significantly decreased CD144+/α-SMA+ double-positive cell percentages and reduced mean α-SMA expression compared to native controls (Figure 1; **p<0.0001).
Conclusions: This study shows that both drugs have anti-proliferative effects, but more importantly, significantly inhibit fibrogenic activation of human EEC. These findings support their potential therapeutic benefit in treating fibroelastic disease and suggest that their beneficial clinical effects may originate from suppression of fibroelastic remodeling at the endocardial level.
Hypothesis: Imatinib mesylate and sirolimus safely and effectively suppress fibrogenic activation of EECs.
Methods: EECs were isolated from fibroelastic cardiac tissue obtained during routine surgeries and grown in culture. Cells were treated with clinically relevant concentrations of imatinib mesylate (0.1, 0.5, 1μM), sirolimus (5, 10, and 20nM), vehicle control (0.1% DMSO), or left untreated (native control). The medium concentrations within the therapeutic range were used for subsequent experiments. Cytotoxicity was assessed using Annexin V/PI staining by flow cytometry at 24 hours, and cell proliferation by MTT assay after 48 hours. Anti-fibrogenic effects were evaluated following 24 hours of TGFβ-1 stimulation (to induce fibrogenic activation) using immunocytochemistry for co-expression of endothelial marker CD144 and mesenchymal marker α-SMA, and mean α-SMA expression. All experiments were performed in biological triplicate. Flow cytometry analysis of CD31 and fibroblast-specific markers was performed to confirm anti-fibrogenic effects.
Results: Imatinib and Sirolimus significantly reduced proliferation compared to native and vehicle controls (*p<0.0002, **p<0.0001) (Table 1). Annexin V/PI staining revealed no significant differences in early or late apoptotic cell populations among treated groups and controls (p=0.83 and p=0.75, respectively). Treatment with both Imatinib and Sirolimus resulted in significantly decreased CD144+/α-SMA+ double-positive cell percentages and reduced mean α-SMA expression compared to native controls (Figure 1; **p<0.0001).
Conclusions: This study shows that both drugs have anti-proliferative effects, but more importantly, significantly inhibit fibrogenic activation of human EEC. These findings support their potential therapeutic benefit in treating fibroelastic disease and suggest that their beneficial clinical effects may originate from suppression of fibroelastic remodeling at the endocardial level.
More abstracts on this topic:
1-year comparison of quadruple therapy sequencing strategies for heart failure with reduced ejection fraction using an individual-based state-transition microsimulation model
Turgeon Ricky, Van Minh Tri, Loewen Peter, Hawkins Nathaniel, Sadatsafavi Mohsen, Zhang Wei, Mackay Kelly
4-Phenylbutyric Acid Reduces Endoplasmic Reticulum Retention and Partially Restores Function of LDLR p.D622N Mutation In Vitro: A Potential Therapy for HypercholesterolemiaWang Yongxiang, Zhang Piyi, Bai Ming, Zhang Zheng