Final ID: MP2219
Lower Risk of Cardiovascular Events in Patients with Clinical Atherosclerotic Cardiovascular Disease Initiated on Semaglutide 2.4 mg in the Real-world: Results from the SCORE-Clinical ASCVD Study (Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity in the Real World – Clinical Atherosclerotic Cardiovascular Disease Population)
Abstract Body (Do not enter title and authors here): Background
In the SELECT trial, semaglutide 2.4 mg significantly reduced the risk of major adverse cardiovascular events (MACE) in patients with established cardiovascular disease (CVD) (myocardial infarction [MI], stroke, and/or peripheral arterial disease [PAD]) and overweight/obesity without diabetes. However, real-world evidence on its effectiveness in reducing MACE outcomes among patients with a broader set of atherosclerotic CVD (ASCVD) conditions (i.e., clinical ASCVD) is limited.
Research Question
To evaluate the association between semaglutide 2.4 mg use and the risk of MACE outcomes among U.S. adults with overweight/obesity and clinical ASCVD (including coronary artery disease, MI, angina, ischemic stroke, transient ischemic attack, carotid or other arterial stenosis, PAD, and coronary or other arterial revascularization) but no diabetes using real-world data.
Methods
Patients aged ≥ 45 years with overweight/obesity and clinical ASCVD but no diabetes were identified from the U.S. Komodo Research Data. A propensity score model including 73 variables was used to match patients (1:2) who did and did not initiate semaglutide 2.4 mg (6/2021 to 12/2024). Hazard ratios for semaglutide 2.4 mg use vs. non-use were derived using Cox proportional hazards models for the revised 3-point MACE (MI, stroke, and all-cause mortality), revised 5-point MACE (including revised 3-point MACE, hospitalization for heart failure [HF], and coronary revascularization), and 3-point MACE and 5-point MACE (replacing all-cause mortality with cardiovascular [CV]-related mortality).
Results
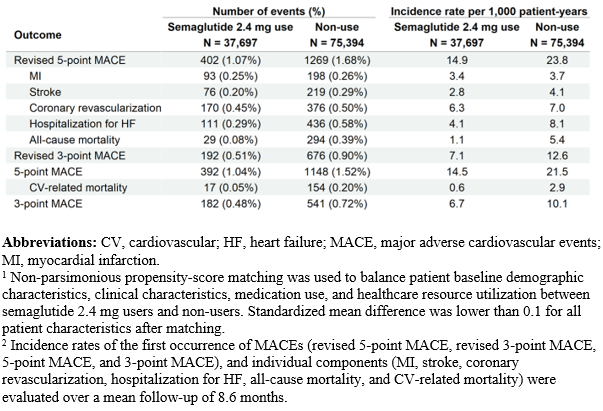
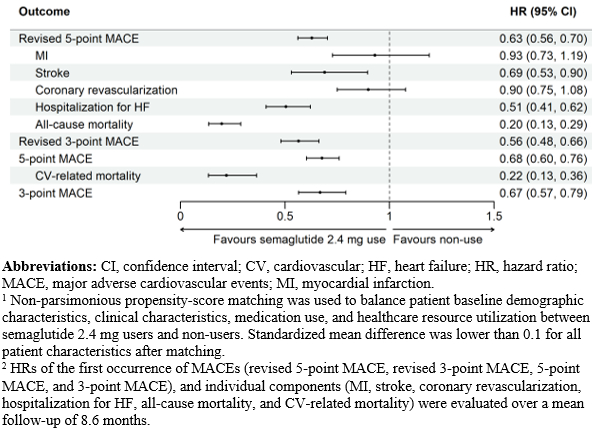
After matching, 37,697 patients who initiated semaglutide 2.4 mg and 75,394 non-users were included; characteristics were well-balanced (standardized mean difference <0.1 for all). Compared with non-use, semaglutide 2.4 mg use was associated with a lower risk of revised 5-point MACE (hazard ratio [HR]: 0.63, 95% confidence interval [CI]: 0.56-0.70), revised 3-point MACE (HR: 0.56, 95% CI: 0.48 – 0.66), 5-point MACE (HR: 0.68, 95% CI: 0.60-0.76), and 3-point MACE (HR: 0.67, 95% CI: 0.57-0.79) (all p < 0.001). Semaglutide 2.4 mg use was also associated with lower rates of stroke, hospitalization for HF, all-cause mortality, and CV-related mortality, compared to non-use.
Conclusion
In this real-world study of U.S. patients with overweight/obesity and clinical ASCVD without diabetes, semaglutide 2.4 mg use was associated with significantly lower risks of MACE outcomes.
In the SELECT trial, semaglutide 2.4 mg significantly reduced the risk of major adverse cardiovascular events (MACE) in patients with established cardiovascular disease (CVD) (myocardial infarction [MI], stroke, and/or peripheral arterial disease [PAD]) and overweight/obesity without diabetes. However, real-world evidence on its effectiveness in reducing MACE outcomes among patients with a broader set of atherosclerotic CVD (ASCVD) conditions (i.e., clinical ASCVD) is limited.
Research Question
To evaluate the association between semaglutide 2.4 mg use and the risk of MACE outcomes among U.S. adults with overweight/obesity and clinical ASCVD (including coronary artery disease, MI, angina, ischemic stroke, transient ischemic attack, carotid or other arterial stenosis, PAD, and coronary or other arterial revascularization) but no diabetes using real-world data.
Methods
Patients aged ≥ 45 years with overweight/obesity and clinical ASCVD but no diabetes were identified from the U.S. Komodo Research Data. A propensity score model including 73 variables was used to match patients (1:2) who did and did not initiate semaglutide 2.4 mg (6/2021 to 12/2024). Hazard ratios for semaglutide 2.4 mg use vs. non-use were derived using Cox proportional hazards models for the revised 3-point MACE (MI, stroke, and all-cause mortality), revised 5-point MACE (including revised 3-point MACE, hospitalization for heart failure [HF], and coronary revascularization), and 3-point MACE and 5-point MACE (replacing all-cause mortality with cardiovascular [CV]-related mortality).
Results
After matching, 37,697 patients who initiated semaglutide 2.4 mg and 75,394 non-users were included; characteristics were well-balanced (standardized mean difference <0.1 for all). Compared with non-use, semaglutide 2.4 mg use was associated with a lower risk of revised 5-point MACE (hazard ratio [HR]: 0.63, 95% confidence interval [CI]: 0.56-0.70), revised 3-point MACE (HR: 0.56, 95% CI: 0.48 – 0.66), 5-point MACE (HR: 0.68, 95% CI: 0.60-0.76), and 3-point MACE (HR: 0.67, 95% CI: 0.57-0.79) (all p < 0.001). Semaglutide 2.4 mg use was also associated with lower rates of stroke, hospitalization for HF, all-cause mortality, and CV-related mortality, compared to non-use.
Conclusion
In this real-world study of U.S. patients with overweight/obesity and clinical ASCVD without diabetes, semaglutide 2.4 mg use was associated with significantly lower risks of MACE outcomes.
More abstracts on this topic:
30-day and one-year outcomes of patients with severe aortic stenosis after TAVI using Myval : A Meta-analysis
Hasabo Elfatih A., Sultan Sherif, Soliman Osama, A. Aboali Amira, Hemmeda Lina, Salah Alaa, Alrawa Salma S., Elgadi Ammar, Abdalmotalib Malaz, Yasir H Eissa Abdullatif, Mahmmoud Fadelallah Eljack Mohammed
ApoB-100 peptide nanoparticles inhibit established atherosclerosis progression in female HLA-A*0201 transgenic miceZhou Jianchang, Zhao Xiaoning, Dimayuga Paul, Lio Nicole, Cercek Bojan, Trac Noah, Chung Eun Ji, Shah Prediman, Chyu Kuang-yuh