Final ID: MP488
Real-World Risk Reduction of Cardiometabolic Comorbidities and Improvement of Biomarkers among Patients with Overweight or Obesity Treated with Semaglutide 2.4 mg
Abstract Body (Do not enter title and authors here): Introduction
Semaglutide 2.4 mg has been shown to reduce the risk and severity of cardiometabolic comorbidities (CMCs) and to improve cardiometabolic biomarkers in patients with overweight or obesity in clinical trials. However, real-world evidence in these areas is limited.
Research Question
This study compared the incidence rates of CMCs and 12-month changes in biomarkers between adults with overweight/obesity with and without once-weekly subcutaneous semaglutide 2.4 mg use.
Methods
A retrospective cohort study using a large U.S. claims database linked with clinical data evaluated patients with semaglutide 2.4 mg use for ≥12 months (June 2021–June 2023) and those without. For each CMC, including hypertension, T2D, dyslipidemia, HF, prediabetes, ASCVD, and CVD, patients were excluded from analysis of each individual CMC if they had claims indicating the corresponding CMC during the 12-month baseline. For each biomarker, patients were required to have the corresponding biomarker measurements at both baseline and 12-month follow-up. For each CMC and biomarker, propensity score weighting was used to balance patient characteristics between semaglutide 2.4 mg users and non-users. Time to CMCs were compared using weighted Cox proportional hazards models and biomarker changes were compared using weighted linear regression.
Results
Sample sizes ranged from 14,067 to 25,490 in semaglutide 2.4 mg users and 4,271,926 to 8,578,948 in non-users across CMCs. After balancing, baseline characteristics were similar between users and non-users. Mean age ranged from 44.4 to 47.2 years among users and 44.4 to 47.4 years among non-users; 77%–83% of users and 75%–82% of non-users were women. Compared to non-users, semaglutide 2.4 mg users had significantly lower risk of incident hypertension (Hazard ratio [HR]: 0.62, 95% confidence intervals [CI]: 0.57; 0.68), T2D (HR: 0.45, 95% CI: 0.39; 0.51), HF (HR: 0.48, 95% CI: 0.38; 0.61), prediabetes (HR: 0.75, 95% CI: 0.70; 0.80), ASCVD (HR: 0.65, 95% CI: 0.58; 0.72), and CVD (HR: 0.69, 95% CI: 0.64; 0.75) (all p<0.001). The risk of dyslipidemia was similar between users and non-users (HR: 1.05, 95% CI: 1.00; 1.11, p=0.05).
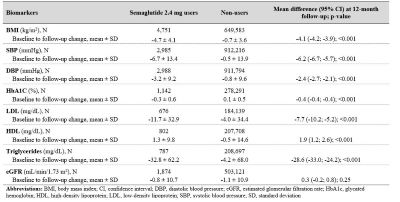
Semaglutide 2.4 mg use was also associated with significant improvements in cardiometabolic biomarkers specified in Table 1 and slower decline in eGFR.
Conclusion
In adults with overweight/obesity, semaglutide 2.4 mg was associated with lower risk of specified CMCs and greater improvements in cardiometabolic biomarkers.
Semaglutide 2.4 mg has been shown to reduce the risk and severity of cardiometabolic comorbidities (CMCs) and to improve cardiometabolic biomarkers in patients with overweight or obesity in clinical trials. However, real-world evidence in these areas is limited.
Research Question
This study compared the incidence rates of CMCs and 12-month changes in biomarkers between adults with overweight/obesity with and without once-weekly subcutaneous semaglutide 2.4 mg use.
Methods
A retrospective cohort study using a large U.S. claims database linked with clinical data evaluated patients with semaglutide 2.4 mg use for ≥12 months (June 2021–June 2023) and those without. For each CMC, including hypertension, T2D, dyslipidemia, HF, prediabetes, ASCVD, and CVD, patients were excluded from analysis of each individual CMC if they had claims indicating the corresponding CMC during the 12-month baseline. For each biomarker, patients were required to have the corresponding biomarker measurements at both baseline and 12-month follow-up. For each CMC and biomarker, propensity score weighting was used to balance patient characteristics between semaglutide 2.4 mg users and non-users. Time to CMCs were compared using weighted Cox proportional hazards models and biomarker changes were compared using weighted linear regression.
Results
Sample sizes ranged from 14,067 to 25,490 in semaglutide 2.4 mg users and 4,271,926 to 8,578,948 in non-users across CMCs. After balancing, baseline characteristics were similar between users and non-users. Mean age ranged from 44.4 to 47.2 years among users and 44.4 to 47.4 years among non-users; 77%–83% of users and 75%–82% of non-users were women. Compared to non-users, semaglutide 2.4 mg users had significantly lower risk of incident hypertension (Hazard ratio [HR]: 0.62, 95% confidence intervals [CI]: 0.57; 0.68), T2D (HR: 0.45, 95% CI: 0.39; 0.51), HF (HR: 0.48, 95% CI: 0.38; 0.61), prediabetes (HR: 0.75, 95% CI: 0.70; 0.80), ASCVD (HR: 0.65, 95% CI: 0.58; 0.72), and CVD (HR: 0.69, 95% CI: 0.64; 0.75) (all p<0.001). The risk of dyslipidemia was similar between users and non-users (HR: 1.05, 95% CI: 1.00; 1.11, p=0.05).
Semaglutide 2.4 mg use was also associated with significant improvements in cardiometabolic biomarkers specified in Table 1 and slower decline in eGFR.
Conclusion
In adults with overweight/obesity, semaglutide 2.4 mg was associated with lower risk of specified CMCs and greater improvements in cardiometabolic biomarkers.
More abstracts on this topic:
Adiposomal microRNAs Mediate Vascular Dysfunction in Obesity-Associated Type 2 Diabetes
Mirza Imaduddin, Morsy Mohammed, Levitan Irena, Raj Usha, Mahmoud Abeer
Adiposity and Cardiac Function in South Asian Americans: Findings from the MASALA StudyKanaya Alka, Nelson Lauren, Running Allison, Lin Feng, Kandula Namratha, Gadgil Meghana, Win Sithu, Shah Sanjiv