Final ID: MP2494
Lower Risk of Cardiovascular Events in Patients with Heart Failure Initiated on Semaglutide 2.4 mg in the Real-world: Results from the SCORE-HF Study (Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity in the Real World – Heart Failure Population)
Abstract Body (Do not enter title and authors here): Background
In a prespecified analysis of the SELECT trial, semaglutide 2.4 mg reduced major adverse cardiovascular events (MACE) and heart failure (HF) composite outcomes in patients with established cardiovascular disease, overweight/obesity, and prevalent HF without diabetes. However, real-world evidence on the effectiveness of semaglutide 2.4 mg in reducing MACE and HF outcomes among patients with HF remains limited.
Research Questions
To evaluate the associations between semaglutide 2.4 mg use and the risk of MACE and HF outcomes, respectively, among U.S. adults with overweight/obesity and HF but no diabetes in routine clinical practice using real-world data.
Methods
Patients aged ≥45 years with overweight/obesity and HF but no diabetes were identified from a large claims database linked to clinical and laboratory data (Komodo Research Data). A propensity score model including 64 variables was used to match patients (1:2) who did and did not initiate semaglutide 2.4 mg (6/2021-12/2024). Hazard ratios for semaglutide 2.4 mg use vs. non-use were derived using Cox proportional hazards models for the revised 3-point MACE (myocardial infarction, stroke, and all-cause mortality), revised 5-point MACE (including revised 3-point MACE, hospitalization for HF, and coronary revascularization), 3-point MACE and 5-point MACE (replacing all-cause mortality with cardiovascular [CV]-related mortality), and 3-point HF outcome (hospitalization for HF, urgent visit for HF, and CV-related mortality).
Results
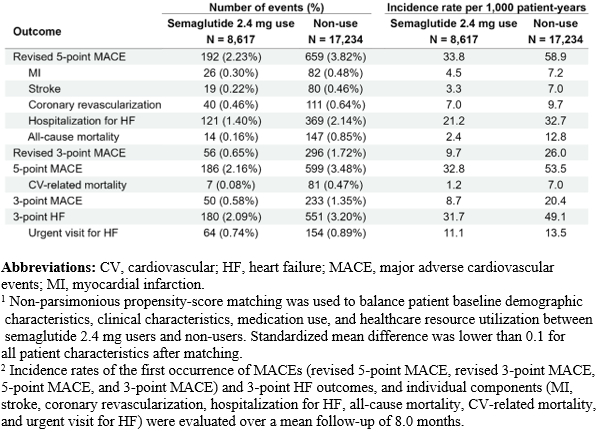
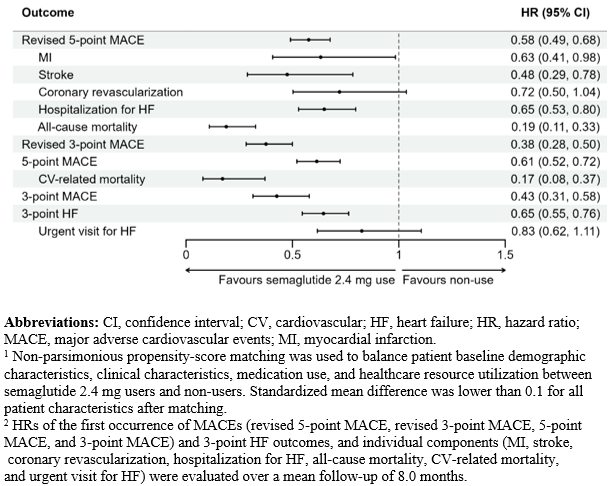
After matching, 8,617 patients who initiated semaglutide 2.4 mg and 17,234 non-users were included; characteristics were well-balanced (standardized mean difference <0.1 for all). Compared with non-use, semaglutide 2.4 mg use was associated with a lower risk of revised 5-point MACE (hazard ratio [HR]: 0.58, 95% confidence interval [CI]: 0.49 – 0.68), revised 3-point MACE (HR: 0.38, 95% CI: 0.28 – 0.50), 5-point MACE (HR: 0.61, 95% CI: 0.52 – 0.72), 3-point MACE (HR: 0.43, 95% CI: 0.31 – 0.58), and 3-point HF (HR: 0.65, 95% CI: 0.55 – 0.76) (all p < 0.001). Semaglutide 2.4 mg use was also associated with lower risk of MI, stroke, hospitalization for HF, all-cause mortality, and CV-related mortality, compared to non-use.
Conclusion
In this real-world study of U.S. patients with overweight/obesity and HF but no diabetes, semaglutide 2.4 mg use was associated with significantly lower risks of MACE and HF outcomes.
In a prespecified analysis of the SELECT trial, semaglutide 2.4 mg reduced major adverse cardiovascular events (MACE) and heart failure (HF) composite outcomes in patients with established cardiovascular disease, overweight/obesity, and prevalent HF without diabetes. However, real-world evidence on the effectiveness of semaglutide 2.4 mg in reducing MACE and HF outcomes among patients with HF remains limited.
Research Questions
To evaluate the associations between semaglutide 2.4 mg use and the risk of MACE and HF outcomes, respectively, among U.S. adults with overweight/obesity and HF but no diabetes in routine clinical practice using real-world data.
Methods
Patients aged ≥45 years with overweight/obesity and HF but no diabetes were identified from a large claims database linked to clinical and laboratory data (Komodo Research Data). A propensity score model including 64 variables was used to match patients (1:2) who did and did not initiate semaglutide 2.4 mg (6/2021-12/2024). Hazard ratios for semaglutide 2.4 mg use vs. non-use were derived using Cox proportional hazards models for the revised 3-point MACE (myocardial infarction, stroke, and all-cause mortality), revised 5-point MACE (including revised 3-point MACE, hospitalization for HF, and coronary revascularization), 3-point MACE and 5-point MACE (replacing all-cause mortality with cardiovascular [CV]-related mortality), and 3-point HF outcome (hospitalization for HF, urgent visit for HF, and CV-related mortality).
Results
After matching, 8,617 patients who initiated semaglutide 2.4 mg and 17,234 non-users were included; characteristics were well-balanced (standardized mean difference <0.1 for all). Compared with non-use, semaglutide 2.4 mg use was associated with a lower risk of revised 5-point MACE (hazard ratio [HR]: 0.58, 95% confidence interval [CI]: 0.49 – 0.68), revised 3-point MACE (HR: 0.38, 95% CI: 0.28 – 0.50), 5-point MACE (HR: 0.61, 95% CI: 0.52 – 0.72), 3-point MACE (HR: 0.43, 95% CI: 0.31 – 0.58), and 3-point HF (HR: 0.65, 95% CI: 0.55 – 0.76) (all p < 0.001). Semaglutide 2.4 mg use was also associated with lower risk of MI, stroke, hospitalization for HF, all-cause mortality, and CV-related mortality, compared to non-use.
Conclusion
In this real-world study of U.S. patients with overweight/obesity and HF but no diabetes, semaglutide 2.4 mg use was associated with significantly lower risks of MACE and HF outcomes.
More abstracts on this topic:
A Multimodality Education Model Improves Healthcare Professionals' Competency in Managing Cardiovascular Risk Factors in Type 2 Diabetes: A Mixed-Methods Study
Madhusudhan Divya, Pressley Alyssa, El Sayed Nuha, Okeke Oge, Bradley Sarah, Blanco Caroline, Perla Esteban, Jennings Ruby, Picou Kylie, Mcweeny Patrick, Crabill Carrianne
Accelerometer-derived physical activity, long-term exposure to ambient PM2.5 and risk of cardiovascular disease mortalityYuan Sheng, Lin Zhangyu, Song Yanjun, Dou Kefei