Final ID: MP1823
Association of Plasma Transthyretin Levels with Risk of Heart Failure in a Multi-Ancestry Population
Abstract Body (Do not enter title and authors here):
Background: Transthyretin (TTR) amyloidosis is a progressive disorder driven by destabilization of the TTR tetramer, leading to amyloid fibril deposition. Lower circulating TTR levels, a marker of TTR instability, have been linked to increased heart failure (HF) risk, primarily in European ancestry populations. TTR gene variants affecting plasma TTR concentrations have shown similar associations. However, it is unknown whether these findings extend to diverse, multi-ancestry populations.
Research Question: Are lower plasma TTR levels associated with increased risk of HF in multi-ancestry populations?
Methods: This prospective study included multiethnic US adults aged ≥18 from the All of Us Research Program with TTR levels between 5 and 45 mg/dL. The primary outcome was HF. Secondarily, the study assessed whether TTR gene variants associated with tetramer instability were linked to lower plasma TTR concentrations and increased HF risk. Multivariable adjusted Cox regression models, using age as the time scale and adjusted for sex and genetic ancestry were used to assess the risk of HF.
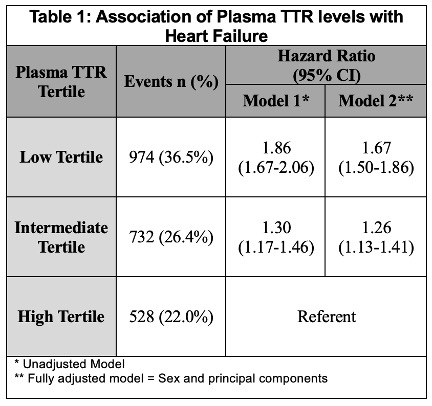
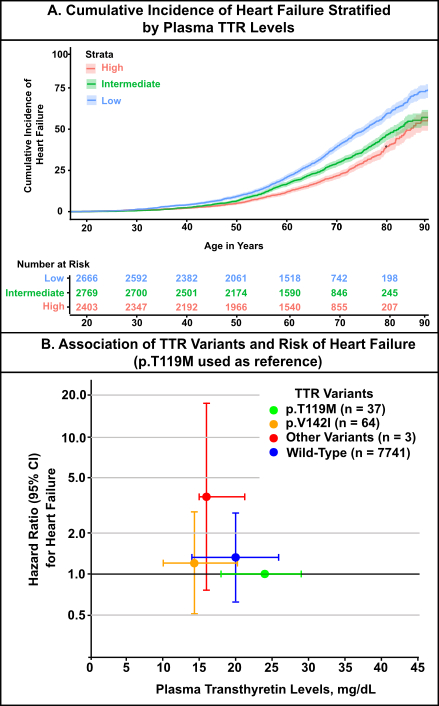
Results: The study population was ancestrally diverse, with 67.4% of patients of European ancestry and 32.6% representing non-European ancestries, including African (21.2%), Admixed (9.7%), East Asian (0.9%), South Asian (0.5%), and Middle Eastern (0.3%) backgrounds. Patients were stratified into tertiles based on plasma TTR levels: high (28.00 mg/dL [IQR 26.00–31.30], n=2,405), intermediate (20.80 mg/dL [18.60–22.50], n=2,770), and low (11.50 mg/dL [9.00–14.00], n=2,670). Compared to individuals in the highest tertile of TTR levels, those in the lowest tertile had a significantly increased risk of HF (HRadj: 1.55 [95% CI, 1.39–1.73]), while those in the intermediate tertile had a moderately elevated risk (HRadj: 1.23 [95% CI, 1.10–1.38]). (Table 1; Figure A) Using the most stable TTR variant (p.T119M) as the reference group, all other genotypes were associated with a higher risk of HF: p.V142I (HR: 1.2 [95% CI 0.5–2.8]), wild-type (HR: 1.3 [95% CI 0.6–2.8]), and non-p.V142I variants (HR 3.6, 95% CI 0.8–17.6). (Figure B)
Conclusion: Lower plasma TTR levels were independently associated with increased HF risk in a large, diverse cohort. Genotype-based differences in TTR stability were directionally consistent with HF risk, supporting the biologic link between TTR instability and cardiac dysfunction. These findings warrant further studies to confirm the observed associations.
Background: Transthyretin (TTR) amyloidosis is a progressive disorder driven by destabilization of the TTR tetramer, leading to amyloid fibril deposition. Lower circulating TTR levels, a marker of TTR instability, have been linked to increased heart failure (HF) risk, primarily in European ancestry populations. TTR gene variants affecting plasma TTR concentrations have shown similar associations. However, it is unknown whether these findings extend to diverse, multi-ancestry populations.
Research Question: Are lower plasma TTR levels associated with increased risk of HF in multi-ancestry populations?
Methods: This prospective study included multiethnic US adults aged ≥18 from the All of Us Research Program with TTR levels between 5 and 45 mg/dL. The primary outcome was HF. Secondarily, the study assessed whether TTR gene variants associated with tetramer instability were linked to lower plasma TTR concentrations and increased HF risk. Multivariable adjusted Cox regression models, using age as the time scale and adjusted for sex and genetic ancestry were used to assess the risk of HF.
Results: The study population was ancestrally diverse, with 67.4% of patients of European ancestry and 32.6% representing non-European ancestries, including African (21.2%), Admixed (9.7%), East Asian (0.9%), South Asian (0.5%), and Middle Eastern (0.3%) backgrounds. Patients were stratified into tertiles based on plasma TTR levels: high (28.00 mg/dL [IQR 26.00–31.30], n=2,405), intermediate (20.80 mg/dL [18.60–22.50], n=2,770), and low (11.50 mg/dL [9.00–14.00], n=2,670). Compared to individuals in the highest tertile of TTR levels, those in the lowest tertile had a significantly increased risk of HF (HRadj: 1.55 [95% CI, 1.39–1.73]), while those in the intermediate tertile had a moderately elevated risk (HRadj: 1.23 [95% CI, 1.10–1.38]). (Table 1; Figure A) Using the most stable TTR variant (p.T119M) as the reference group, all other genotypes were associated with a higher risk of HF: p.V142I (HR: 1.2 [95% CI 0.5–2.8]), wild-type (HR: 1.3 [95% CI 0.6–2.8]), and non-p.V142I variants (HR 3.6, 95% CI 0.8–17.6). (Figure B)
Conclusion: Lower plasma TTR levels were independently associated with increased HF risk in a large, diverse cohort. Genotype-based differences in TTR stability were directionally consistent with HF risk, supporting the biologic link between TTR instability and cardiac dysfunction. These findings warrant further studies to confirm the observed associations.
More abstracts on this topic:
A Case of Concomitant Wild-Type Transthyretin and Systemic Light Chain Amyloidosis Involving Separate Organs
Chiu Leonard, Afrough Aimaz, Nadeem Urooba, Jebakumar Deborah, Grodin Justin
A Predictive Tool and Diagnostic Screening Algorithm for the Identification of Transthyretin Amyloid Cardiomyopathy in High-Risk Patient PopulationsChai Jocelyn, Sathananthan Janarthanan, Fine Nowell, Davis Margot, Starovoytov Andrew, Campbell Christine, Hawkins Nathaniel, Virani Sean, Luong Michael, Straatman Lynn, Kiess Marla, Worsley Daniel