Final ID: MP973
A Phase I, Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Single Ascending Doses of N-acetylgalactosamine Small Interfering RNA Conjugate, BPR-30221616, in Healthy Participants, for Potential Treatment of Transthyretin Amyloidosis
Abstract Body (Do not enter title and authors here): Introduction: Transthyretin amyloidosis (ATTR) is a progressive disease characterized by abnormal buildup amyloid of misfolded transthyretin (TTR) protein in multiple organs and the major disease manifestations include polyneuropathy and cardiomyopathy. The pathogenic amyloid accumulation results in organ dysfunction and reduced life expectancy. BPR-30221616 is an experimental small interfering RNA (siRNA), targeting TTR mRNA to decrease the circulating TTR proteins, therefore mitigating the ATTR symptoms. Here, we present the first-in-human data from the ongoing phase I study of BPR-30221616.
Hypothesis: To evaluate the safety, pharmacokinetics, pharmacodynamics and immunogenicity of single ascending doses of BPR-30221616.
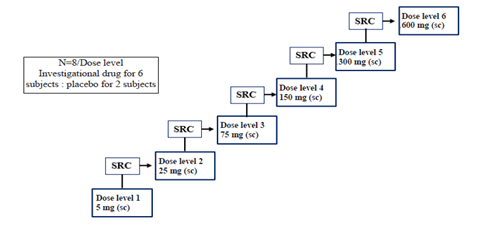
Methods: This ongoing, single-center, Phase I, randomized, double-blind, placebo-controlled study (NCT06760455) is conducted in China in healthy participants aged 18-65 years, with BMI of 18.0-30.0 kg/m2 and body weight ≥50.0 kg for males and ≥45.0 kg for females. The study recruits six cohorts, each composed of 8 subjects randomized 3:1 for the test drug and placebo respectively. Each cohort receives a single dose of BPR-30221616 (5, 25, 75, 150, 300, or 600 mg) or placebo in a dose-escalation manner (Figure). The pharmacokinetics of the test drug and changes of serum TTR and adverse event (AEs),if any are assessed for up to 360 days.
Results: As of the submission date of this abstract, 24 participants were enrolled in the 5 mg, 25 mg and 75 mg cohorts, and finished the 90-day, 29-day and 8-day visits, respectively. Single dose of BRP-30221616 up to 75 mg were well tolerated, and the majority of AEs were mild. No safety signals, including liver-related signals, were identified. In the 5 mg and 25 mg cohorts, BPR-30221616 was rapidly absorbed with a Tmax of 3-4 h, and exhibited a short plasma elimination half-life of 4-8 h. The onset of reduced serum TTR was observed within 8 days after dosing, and the mean inhibition was 66.6% for 5 mg and 85.0% for 25 mg at day 22. Clinical serum TTR inhibition duration time was under continuous observation, and we observed that a single bolus dose of BPR-30221616 reduces serum TTR at a superior level in nonhuman primate and anticipated this deep and sustained reduction pattern is well translated into the human subjects.
Conclusion: BPR-30221616 exhibits rapid and sustained knockdown of serum TTR at low doses, indicating its potential as a siRNA therapeutic for transthyretin amyloidosis.
Hypothesis: To evaluate the safety, pharmacokinetics, pharmacodynamics and immunogenicity of single ascending doses of BPR-30221616.
Methods: This ongoing, single-center, Phase I, randomized, double-blind, placebo-controlled study (NCT06760455) is conducted in China in healthy participants aged 18-65 years, with BMI of 18.0-30.0 kg/m2 and body weight ≥50.0 kg for males and ≥45.0 kg for females. The study recruits six cohorts, each composed of 8 subjects randomized 3:1 for the test drug and placebo respectively. Each cohort receives a single dose of BPR-30221616 (5, 25, 75, 150, 300, or 600 mg) or placebo in a dose-escalation manner (Figure). The pharmacokinetics of the test drug and changes of serum TTR and adverse event (AEs),if any are assessed for up to 360 days.
Results: As of the submission date of this abstract, 24 participants were enrolled in the 5 mg, 25 mg and 75 mg cohorts, and finished the 90-day, 29-day and 8-day visits, respectively. Single dose of BRP-30221616 up to 75 mg were well tolerated, and the majority of AEs were mild. No safety signals, including liver-related signals, were identified. In the 5 mg and 25 mg cohorts, BPR-30221616 was rapidly absorbed with a Tmax of 3-4 h, and exhibited a short plasma elimination half-life of 4-8 h. The onset of reduced serum TTR was observed within 8 days after dosing, and the mean inhibition was 66.6% for 5 mg and 85.0% for 25 mg at day 22. Clinical serum TTR inhibition duration time was under continuous observation, and we observed that a single bolus dose of BPR-30221616 reduces serum TTR at a superior level in nonhuman primate and anticipated this deep and sustained reduction pattern is well translated into the human subjects.
Conclusion: BPR-30221616 exhibits rapid and sustained knockdown of serum TTR at low doses, indicating its potential as a siRNA therapeutic for transthyretin amyloidosis.
More abstracts on this topic:
A DHX38 Spliceosomal Mutation Impairs MYC Signaling, Cardiac Transcriptome Splicing, and Leads to Diastolic Dysfunction
Iwanski Jessika, Sarvagalla Sailu, Methawasin Mei, Van Den Berg Marloes, Churko Jared
A-band titin-truncating variant promotes the development of arrhythmia-induced cardiomyopathy in a novel genetically-engineered porcine modelLee Kwonjae, Del Rio Carlos, Mcnally Elizabeth, Pfenniger Anna, Bhatnagar Ashita, Glinton Kristofor, Burrell Amy, Ober Rebecca, Mcluckie Alicia, Bishop Brian, Rogers Christopher, Geist Gail