Final ID: MP1814
Acoramidis Improved Clinical Outcomes, Function, Quality of Life and NT-proBNP in Patients With Transthyretin Amyloid Cardiomyopathy Regardless of Atrial Fibrillation Status at Baseline
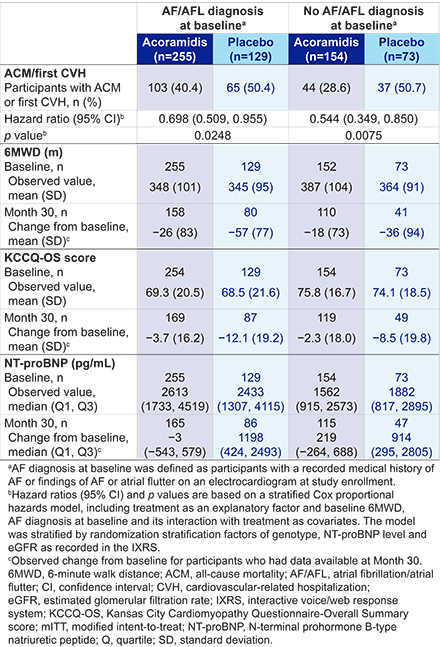
Research Question: How does acoramidis treatment affect ACM or first CVH, 6MWD, KCCQ-OS scores, and NT-proBNP levels at Month 30 in participants with ATTR-CM with or without AF/AFL diagnosis at baseline?
Methods: The ATTRibute-CM study design has been previously published. This post hoc analysis included participants in the modified intention-to-treat population (N=611) grouped by AF/AFL diagnosis at baseline (defined as recorded AF medical history or the presence of AF or AFL on an ECG at enrollment). The rate of ACM or first CVH at Month 30 was compared between groups using a stratified Cox proportional hazards model. Changes from baseline to Month 30 in 6MWD, KCCQ-OS scores and NT-proBNP levels were summarized descriptively.
Results: Overall, 62.8% (384/611) of participants had an AF/AFL diagnosis at baseline (acoramidis: 255/409, placebo: 129/202). These participants had lower mean 6MWD and KCCQ-OS scores and higher median NT-proBNP levels than those without AF/AFL diagnosis at baseline. Treatment with acoramidis reduced ACM or first CVH, slowed the decline in 6MWD and KCCQ-OS scores and blunted the rise in NT-proBNP compared with placebo, regardless of AF/AFL diagnosis at baseline (Table).
Conclusions: Acoramidis improved clinical outcomes (ACM, CVH), functional status, QoL and NT-proBNP levels relative to placebo in participants with ATTR-CM, regardless of AF/AFL diagnosis at baseline.
- Sperry, Brett ( Saint Luke’s Mid America Heart Institute and the University of Missouri-Kansas City , Kansas City , Missouri , United States )
- Tamby, Jean-francois ( BridgeBio Pharma, Inc. , San Francisco , California , United States )
- Castano, Adam ( BridgeBio Pharma, Inc. , San Francisco , California , United States )
- Fox, Jonathan ( BridgeBio Pharma, Inc. , San Francisco , California , United States )
- Cheng, Richard ( University of Washington , Seattle , Washington , United States )
- Judge, Daniel ( Medical University of South Carolina , Charleston , South Carolina , United States )
- Cappelli, Francesco ( Careggi University Hospital , Florence , Italy )
- Masri, Ahmad ( Oregon Health Sciences University , Portland , Oregon , United States )
- Grogan, Martha ( Mayo Clinic , Rochester , Minnesota , United States )
- Mooney, Deirdre ( Providence Center for Advanced Heart Disease & Transplantation , Spokane , Washington , United States )
- Akinboboye, Olakunle ( Laurelton Heart Specialists PC , Rosedale , New York , United States )
- Drachman, Brian ( Penn Presbyterian Medical Center , Philadelphia , Pennsylvania , United States )
- Nativi-nicolau, Jose ( Mayo Clinic , Jacksonville , Florida , United States )
- Kobayashi, Masatake ( Tokyo Medical University , Tokyo , Japan )
- Chen, Chris ( BridgeBio Pharma, Inc. , San Francisco , California , United States )
Meeting Info:
Session Info:
Contemporary Cardiac Amyloidosis Research
Sunday, 11/09/2025 , 03:15PM - 04:30PM
Moderated Digital Poster Session
More abstracts on this topic:
Elbatreek Mahmoud, Goodchild Traci, Lefer David, Evans Lauren, Richardson Thomas, James Rebecca, Schroeder Frederick, Wang Jianhong, Luterman Jim, Gilbert Tonya, Fisher Richard
A Phase I, Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Single Ascending Doses of N-acetylgalactosamine Small Interfering RNA Conjugate, BPR-30221616, in Healthy Participants, for Potential Treatment of Transthyretin AmyloidosisHan Xiaohong, Chen Rui, Cheng Xiwen, Zhang Langxi, Huang Haoxi, Fan Shengjun
More abstracts from these authors:
Witteles Ronald, Mitter Sumeet, Gillmore Julian, Hanna Mazen, Berk John, Mitchell Joshua, Shah Keyur, Kobayashi Masatake, Xiong Kuangnan, Castano Adam, Tamby Jean-francois, Fox Jonathan
Serum Transthyretin Levels at Day 28 are Associated with Cardiovascular Outcomes: Insights From the ATTRibute-CM StudySarswat Nitasha, Ruberg Frederick, Chen Chris, Ji Alan, Tamby Jean-francois, Sinha Uma, Fox Jonathan, Maurer Mathew, Cheng Richard, Ambardekar Amrut, Wright Richard, Davis Margot, Gillmore Julian, Grodin Justin, Mitchell Joshua, Mooney Deirdre, Nativi-nicolau Jose