Final ID: MP1817
Serum Transthyretin Levels at Day 28 are Associated with Cardiovascular Outcomes: Insights From the ATTRibute-CM Study
Hypothesis: Acoramidis-mediated early increase in sTTR levels ≥20 mg/dL (lower limit of normal) at Day 28 reduces risks of CVM and cardiovascular-related hospitalizations (CVH) in patients with ATTR-CM.
Methods: Analyses were conducted in the ATTRibute-CM modified intention-to-treat (mITT) population (acoramidis: 409; placebo: 202). The sTTR concentrations (normal range is 20-40 mg/dL) were assessed using an immunoturbidimetric method (Abbott ARCHITECT system) in a central laboratory. The proportion of participants with sTTR levels below normal at baseline and Day 28 were determined. The relationship between sTTR levels <20 or ≥20 mg/dL, at Day 28 across the pooled acoramidis and placebo treatment groups, and subsequent risk of CVM and of CVH over 30 months were analyzed using a stratified log-rank test.
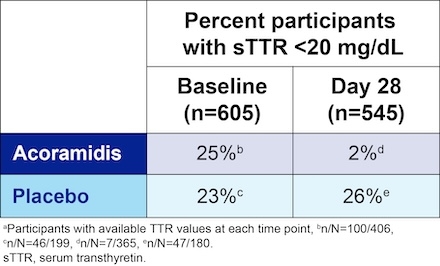
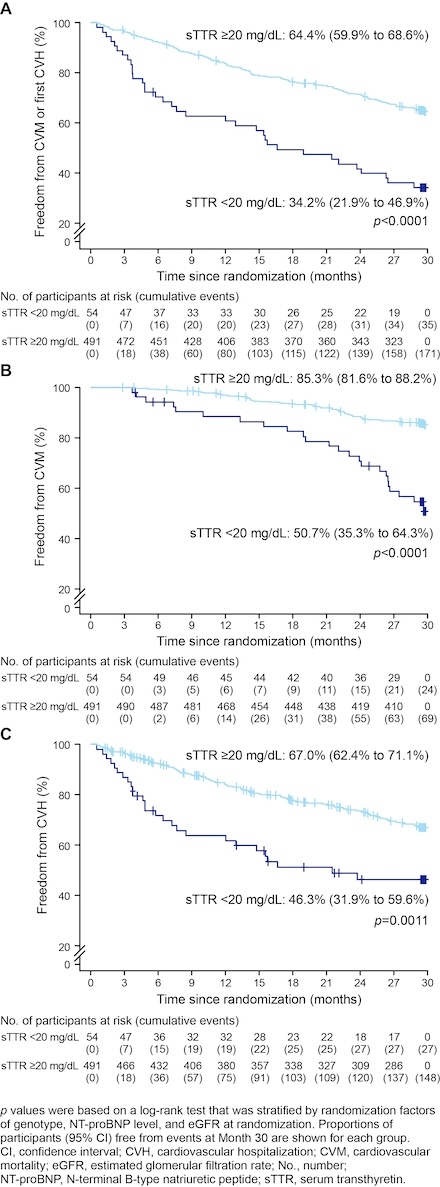
Results: At baseline about 25% of study participants had sTTR levels below normal in both treatment groups. At Day 28, less than 2% of participants had sTTR levels <20 mg/dL in the acoramidis arm compared with 26% in the placebo arm (Table 1). When acoramidis and placebo treatment groups were pooled based on sTTR levels <20 or ≥20 mg/dL at Day 28, participants with sTTR ≥20 mg/dL were associated with a lower risk of the composite endpoint of CVM or first CVH at Month 30 in comparison with <20 mg/dL (p<0.0001) (Figure 1A). Comparable findings were observed for the individual components (CVM, first CVH) (Figure 1B and C).
Conclusion: Across treatment groups, sTTR levels above normal range (≥20 mg/dL) at Day 28 were associated with a lower risk of cardiovascular outcomes at Month 30, when compared with sTTR levels below normal range (<20 mg/dL). Thus, these observations demonstrate that higher sTTR levels over time may have the potential for clinical benefits in both CVM and CVH.
- Sarswat, Nitasha ( University of Chicago Medicine , Chicago , Illinois , United States )
- Ruberg, Frederick ( Boston University Chobanian & Avedisian School of Medicine, Boston Medical Center , Boston , Massachusetts , United States )
- Chen, Chris ( BridgeBio Pharma, Inc , San Francisco , California , United States )
- Ji, Alan ( BridgeBio Pharma, Inc , San Francisco , California , United States )
- Tamby, Jean-francois ( BridgeBio Pharma, Inc , San Francisco , California , United States )
- Sinha, Uma ( BridgeBio Pharma, Inc , San Francisco , California , United States )
- Fox, Jonathan ( BridgeBio Pharma, Inc , San Francisco , California , United States )
- Maurer, Mathew ( Columbia University Vagelos College of Physicians and Surgeons , New York , New York , United States )
- Cheng, Richard ( University of Washington Medicine , Seattle , Washington , United States )
- Ambardekar, Amrut ( University of Colorado , Aurora , Colorado , United States )
- Wright, Richard ( Pacific Heart Institute , Santa Monica , California , United States )
- Davis, Margot ( Vancouver General Hospital , Vancouver , British Columbia , Canada )
- Gillmore, Julian ( University College London, Royal Free Hospital , London , United Kingdom )
- Grodin, Justin ( University of Texas Southwestern Medical Center , Dallas , Texas , United States )
- Mitchell, Joshua ( Washington University School of Medicine , St. Louis , Missouri , United States )
- Mooney, Deirdre ( Providence Center for Advanced Heart Disease & Transplantation , Spokane , Washington , United States )
- Nativi-nicolau, Jose ( Mayo Clinic , Jacksonville , Florida , United States )
Meeting Info:
Session Info:
Contemporary Cardiac Amyloidosis Research
Sunday, 11/09/2025 , 03:15PM - 04:30PM
Moderated Digital Poster Session
More abstracts on this topic:
Han Xiaohong, Chen Rui, Cheng Xiwen, Zhang Langxi, Huang Haoxi, Fan Shengjun
AI-Derived Retinal Vasculature Features Predict Cardiovascular Risk in Patients with Chronic Kidney Disease: Insights from the CRIC StudyDhamdhere Rohan, Modanwal Gourav, Rahman Mahboob, Al-kindi Sadeer, Madabhushi Anant
More abstracts from these authors:
Alexander Kevin, Bhatt Kunal, Judge Daniel, Grodin Justin, Akinboboye Olakunle, Chen Chris, Tamby Jean-francois, Castano Adam, Fox Jonathan, Fontana Marianna, Gillmore Julian, Sarswat Nitasha, Grogan Martha, Solomon Scott, Davis Margot, Cuddy Sarah, Kittleson Michelle, Shah Keyur, Griffin Jan, Ruberg Frederick, Khouri Michel
Acoramidis Improved Clinical Outcomes, Function, Quality of Life and NT-proBNP in Patients With Transthyretin Amyloid Cardiomyopathy Regardless of Atrial Fibrillation Status at BaselineSperry Brett, Tamby Jean-francois, Castano Adam, Fox Jonathan, Cheng Richard, Judge Daniel, Cappelli Francesco, Masri Ahmad, Grogan Martha, Mooney Deirdre, Akinboboye Olakunle, Drachman Brian, Nativi-nicolau Jose, Kobayashi Masatake, Chen Chris