Final ID: MP503
Acoramidis Effect on All-Cause Mortality in Patients with p.V142I (V122I) Variant ATTR-CM: Findings From the ATTRibute-CM Study
Transthyretin (TTR) amyloid cardiomyopathy (ATTR-CM) arises from amyloidogenic aggregates of destabilized TTR protein in variant (ATTRv-CM) or acquired wild-type TTR (ATTRwt-CM). The p.V142I (V122I) variant is the most common pathogenic TTR allele in the USA and has higher mortality vs wild type. Acoramidis, an oral TTR stabilizer achieving ≥90% stabilization, is approved in USA, UK, Europe, and Japan for treating ATTR-CM in adults. In ATTRibute-CM (NCT03860935), acoramidis showed a reduction in all-cause mortality (ACM) or first cardiovascular-related hospitalization (CVH) vs placebo (PBO), with consistent benefit in ATTRwt-CM and ATTRv-CM (all variants). We report clinical outcomes in participants with p.V142I ATTRv-CM.
Research Question
What was the efficacy of acoramidis for clinical outcomes (ACM, CVH) in participants with p.V142I ATTRv-CM in ATTRibute-CM?
Methods
In ATTRibute-CM, 632 participants were randomized 2:1 to receive acoramidis HCl 800 mg or PBO twice daily for 30 months. All participants enrolled in open-label extension (OLE) received acoramidis only. Exploratory post hoc analyses were done in the p.V142I ATTRv-CM subgroup. Time-to-event analyses used a stratified Cox proportional hazards model using treatment group as an explanatory factor and baseline 6MWD as a covariate, stratified by randomization factors: serum NT-proBNP and eGFR. ACM was analyzed at Month 42 (ATTRibute-CM 30 months + 12 months OLE).
Results
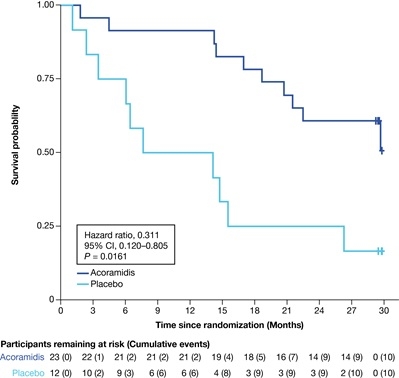
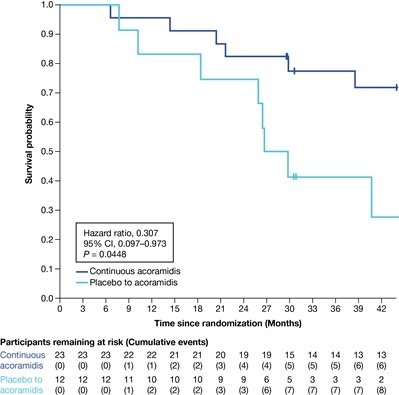
Of the 59 patients with ATTRv-CM reported at randomization, 35 (59.3%) had p.V142I (23 acoramidis, 12 PBO) with comparable baseline characteristics; 4 were homozygous for p.V142I (1 acoramidis, 3 PBO). Through Month 30, ACM/first CVH occurred in 43.5% (10/23) of acoramidis vs 83.3% (10/12) in PBO (HR 0.311; 95% CI 0.120–0.805; Figure 1). Through Month 42, ACM occurred in 26.1% (6/23) of continuous acoramidis vs 66.7% (8/12) in PBO to acoramidis (HR 0.307; 95% CI 0.097–0.973; Figure 2).
Conclusions
In participants with p.V142I ATTRv-CM, acoramidis use was associated with 69% risk reduction in ACM/first CVH through Month 30 and ACM through Month 42 vs PBO. This is the first report of clinical benefit of this magnitude observed in this high-risk population. These findings have biologic plausibility and may reflect the near-complete stabilization observed experimentally with acoramidis in p.V142I ATTR fibrils. These observations require confirmation in larger cohort studies.
- Alexander, Kevin ( Stanford University School of Medicine , Palo Alto , California , United States )
- Bhatt, Kunal ( Emory Healthcare , Atlanta , Georgia , United States )
- Judge, Daniel ( Medical University South Carolina , Charleston , South Carolina , United States )
- Grodin, Justin ( University of Texas Southwestern Medical Center , Dallas , Texas , United States )
- Akinboboye, Olakunle ( Queens Heart Institute , New York , New York , United States )
- Chen, Chris ( BridgeBio Pharma Inc. , San Francisco , California , United States )
- Tamby, Jean-francois ( BridgeBio Pharma Inc. , San Francisco , California , United States )
- Castano, Adam ( BridgeBio Pharma Inc. , San Francisco , California , United States )
- Fox, Jonathan ( BridgeBio Pharma Inc. , San Francisco , California , United States )
- Fontana, Marianna ( University College London , London , United Kingdom )
- Gillmore, Julian ( UCL Centre for Amyloidosis , London , United Kingdom )
- Sarswat, Nitasha ( University of Chicago Medicine , Chicago , Illinois , United States )
- Grogan, Martha ( Mayo Clinic , Rochester , Minnesota , United States )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Davis, Margot ( University of British Columbia , Vancouver , British Columbia , Canada )
- Cuddy, Sarah ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Kittleson, Michelle ( Cedars-Sinai , Los Angeles , California , United States )
- Shah, Keyur ( Virginia Commonwealth University , Richmond , Virginia , United States )
- Griffin, Jan ( Medical University of South Carolina , Charleston , South Carolina , United States )
- Ruberg, Frederick ( Boston University , Boston , Massachusetts , United States )
- Khouri, Michel ( Duke University School of Medicine , Durham , North Carolina , United States )
Meeting Info:
Session Info:
Where Cancer and Cardiovascular Disease Collide: Risks, Disparities, and Evolving Evidence
Saturday, 11/08/2025 , 03:15PM - 04:25PM
Moderated Digital Poster Session
More abstracts on this topic:
Prendergast Heather, Khosla Shaveta, Kitsiou Spyros, Petzel Gimbar Renee, Freels Sally, Sanders Anissa, Daviglus Martha, Carter Barry, Del Rios Marina, Heinert Sara
A Phase I, Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Single Ascending Doses of N-acetylgalactosamine Small Interfering RNA Conjugate, BPR-30221616, in Healthy Participants, for Potential Treatment of Transthyretin AmyloidosisHan Xiaohong, Chen Rui, Cheng Xiwen, Zhang Langxi, Huang Haoxi, Fan Shengjun
More abstracts from these authors:
Davis Margot, Soman Prem, Kittleson Michelle, Berk John, Cao Xiaofan, Tamby Jean-francois, Castano Adam, Fox Jonathan, Shah Keyur, Grogan Martha, Griffin Jan, Sarswat Nitasha, Grodin Justin, Alexander Kevin, Judge Daniel, Gillmore Julian, Cappelli Francesco, Wright Richard
Acoramidis Improved Clinical Outcomes, Function, Quality of Life and NT-proBNP in Patients With Transthyretin Amyloid Cardiomyopathy Regardless of Atrial Fibrillation Status at BaselineSperry Brett, Tamby Jean-francois, Castano Adam, Fox Jonathan, Cheng Richard, Judge Daniel, Cappelli Francesco, Masri Ahmad, Grogan Martha, Mooney Deirdre, Akinboboye Olakunle, Drachman Brian, Nativi-nicolau Jose, Kobayashi Masatake, Chen Chris