Final ID: Sa4086
Itaconate stabilizes ABCA1 to regulate cholesterol burden: insights from targeting with lipid nanoparticles
Abstract Body (Do not enter title and authors here): Background/Aims:
Itaconate (ITA) is a tricarboxylic acid cycle (TCA)-derived metabolite that limits atherosclerotic plaque growth and improves plaque stability via immunomodulation. However, the benefits of ITA may also include lipid metabolism regulation. Here, we utilize plaque-targeting, ITA-conjugated lipid nanoparticles (ITA-LNPs) to study how ITA stabilizes ABCA1, a cholesterol transporter with beneficial effects on atherosclerosis, including cholesterol efflux in plaque macrophages.
Methods/Approach:
Apoe−/− mice were fed a high-cholesterol/high-fat diet (HCHFD) for 12 weeks and injected intravenously once weekly with 50 mg/kg ITA-LNP or Ctrl-LNP (control nanoparticles). Aortas were tested for ITA-LNP biodistribution, quantification of atherosclerotic plaque burden, whole transcriptome analysis via bulk RNA sequencing (RNAseq) and ABCA1 levels. Bone marrow-derived macrophages (BMDMs) and RAW 264.7 cells were treated with ITA-LNP or Ctrl-LNP in the presence of oxLDL, acLDL, or free cholesterol to investigate ITA’s actions on Abca1 expression and ABCA1 stability under a variety of conditions, including stable gene knockdown.
Results/Data:
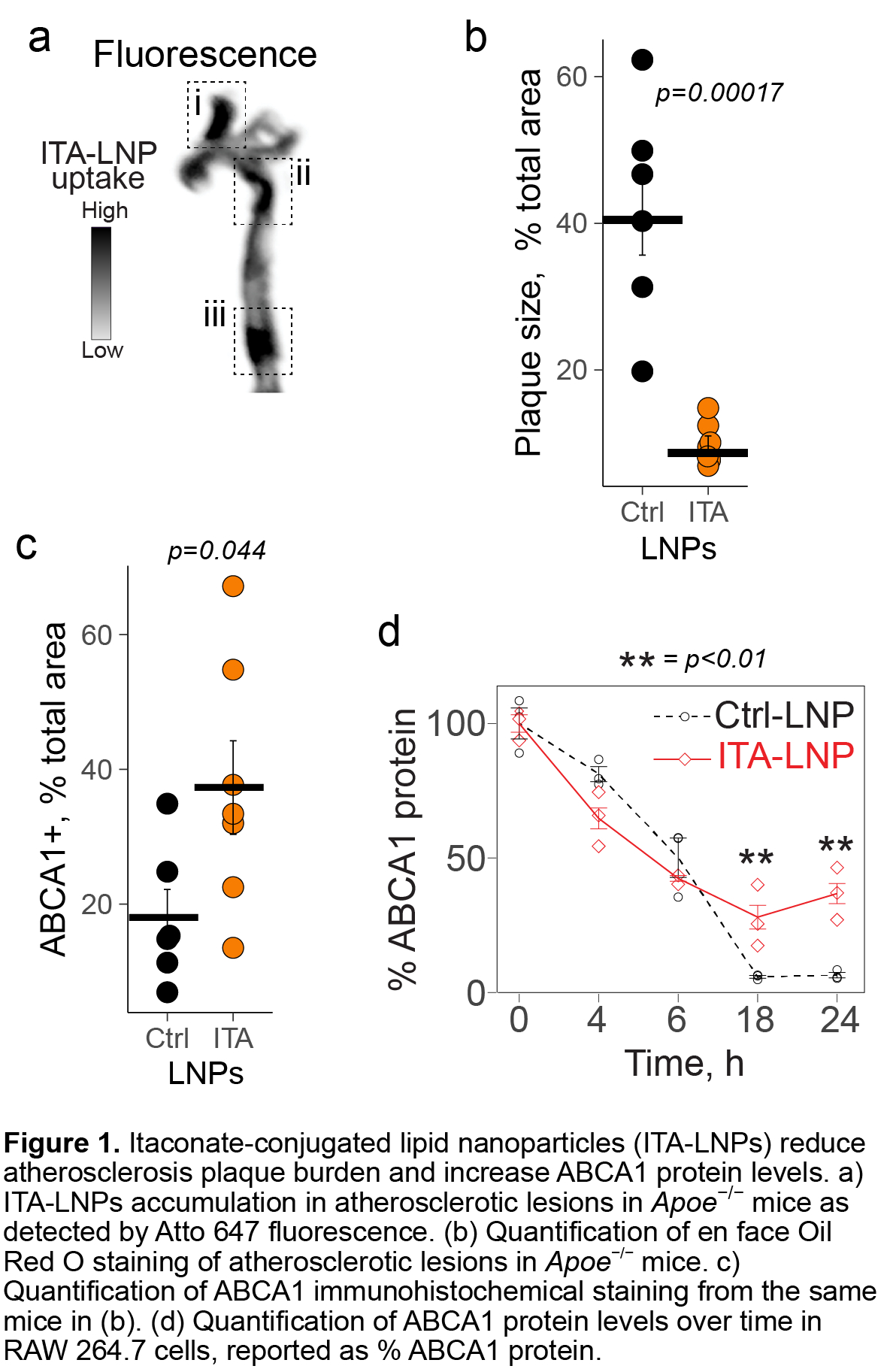
ITA-LNPs, but not Ctrl-LNPs, deposited to atherosclerotic plaque, reduced the plaque size and significantly increased ABCA1 protein levels as seen in branchiocephalic arteries (ITA-LNP = 37.3 ± 6.4% vs 18.0 ± 3.8% of plaque area, p < 0.05, Figure 1a-c), but not at the transcriptional level in whole plaque (via RNAseq, no differentially expressed Abca1 mRNA with FDR<0.05) and in bone marrow-derived macrophages (1.1 ± 0.15 fold vs. 0.73 ± 0.042 fold, p > 0.05). ITA-LNPs prevented ABCA1 decay (82.33 ± 4.78% vs. 28.0 ± 4.4% protein remaining at 18 h after cycloheximide inhibition of de novo protein synthesis, p < 0.01, Figure 1d) via the HO-1-calpain axis and significantly increased cholesterol efflux towards ApoA-I in RAW 264.7 cells (5.31 ± 0.37% vs. 3.09 ± 0.36%, p < 0.001, Figure 1d). This was further confirmed in RAW 264.7 cells with a stable HO-1 knockdown, which did not demonstrate increased ABCA1 stability (28.18 ± 3.05% vs. 33.65 ± 0.30% protein remaining at 18 h, p > 0.05).
Conclusions:
ITA-LNPs regulate lipid metabolism in atherosclerosis by inducing cholesterol efflux. ITA-LNPs are thus an exciting first-in-class experimental nanotherapy with effects on both inflammation and lipid metabolism in atherosclerotic plaque.
Itaconate (ITA) is a tricarboxylic acid cycle (TCA)-derived metabolite that limits atherosclerotic plaque growth and improves plaque stability via immunomodulation. However, the benefits of ITA may also include lipid metabolism regulation. Here, we utilize plaque-targeting, ITA-conjugated lipid nanoparticles (ITA-LNPs) to study how ITA stabilizes ABCA1, a cholesterol transporter with beneficial effects on atherosclerosis, including cholesterol efflux in plaque macrophages.
Methods/Approach:
Apoe−/− mice were fed a high-cholesterol/high-fat diet (HCHFD) for 12 weeks and injected intravenously once weekly with 50 mg/kg ITA-LNP or Ctrl-LNP (control nanoparticles). Aortas were tested for ITA-LNP biodistribution, quantification of atherosclerotic plaque burden, whole transcriptome analysis via bulk RNA sequencing (RNAseq) and ABCA1 levels. Bone marrow-derived macrophages (BMDMs) and RAW 264.7 cells were treated with ITA-LNP or Ctrl-LNP in the presence of oxLDL, acLDL, or free cholesterol to investigate ITA’s actions on Abca1 expression and ABCA1 stability under a variety of conditions, including stable gene knockdown.
Results/Data:
ITA-LNPs, but not Ctrl-LNPs, deposited to atherosclerotic plaque, reduced the plaque size and significantly increased ABCA1 protein levels as seen in branchiocephalic arteries (ITA-LNP = 37.3 ± 6.4% vs 18.0 ± 3.8% of plaque area, p < 0.05, Figure 1a-c), but not at the transcriptional level in whole plaque (via RNAseq, no differentially expressed Abca1 mRNA with FDR<0.05) and in bone marrow-derived macrophages (1.1 ± 0.15 fold vs. 0.73 ± 0.042 fold, p > 0.05). ITA-LNPs prevented ABCA1 decay (82.33 ± 4.78% vs. 28.0 ± 4.4% protein remaining at 18 h after cycloheximide inhibition of de novo protein synthesis, p < 0.01, Figure 1d) via the HO-1-calpain axis and significantly increased cholesterol efflux towards ApoA-I in RAW 264.7 cells (5.31 ± 0.37% vs. 3.09 ± 0.36%, p < 0.001, Figure 1d). This was further confirmed in RAW 264.7 cells with a stable HO-1 knockdown, which did not demonstrate increased ABCA1 stability (28.18 ± 3.05% vs. 33.65 ± 0.30% protein remaining at 18 h, p > 0.05).
Conclusions:
ITA-LNPs regulate lipid metabolism in atherosclerosis by inducing cholesterol efflux. ITA-LNPs are thus an exciting first-in-class experimental nanotherapy with effects on both inflammation and lipid metabolism in atherosclerotic plaque.
More abstracts on this topic:
A 3-Year, Pre-Trial, Real-world Data Analysis of Patients Enrolled in VICTORION-INITIATE: Insights Using Tokenization
Rodriguez Fatima, Cosmatos Irene, Desai Nihar, Wright R, Ross Elsie, Ali Yousuf, Kumar Biswajit, Han Guangyang, Cai Beilei, Abbas Cheryl, Ryan Amy
A Genome-wide CRISPRi Screen Implicates Coronary Artery Disease GWAS Genes as Key Regulators of Adventitial Fibroblast ProliferationJackson William, Zhu Ashley, Gu Wenduo, Berezowitz Alexa, Iyer Meghana, Cheng Paul