Final ID: 4138661
Targeting Arginase I Alleviates Cardiomyopathy
Abstract Body (Do not enter title and authors here): Introduction: Emerging data have shown that pathological cardiac fibrosis and heart failure is driven by recruitment of CD4+ T cells to injured heart tissue. Systemic deletion of CD4+ T cells or splenectomy has been shown to attenuate CD4+ T cell heart infiltration and inflammation. We engineered cargo-less nanoparticles using a biocompatible and biodegradable polymer that target splenic macrophages to overexpress arginase I, leading to local depletion of L-arginine, an amino acid crucial for CD4+ T cell activation and expansion.
Hypothesis: Cargo-less nanoparticles attenuate CD4+ T cell activation, expansion, and heart infiltration in experimental models of cardiomyopathy by regulating cross talk between macrophages and CD4+ T cells via L-arginine depletion in the spleen.
Methods: Spleen-targeting, cargo-less nanoparticles were synthesized from poly(L-lactide-co-glycolide) (PLLGA) and administered to murine and human macrophages in vitro. Arginase I expression and activity were assessed via Western blotting and arginase activity assay. Epigenetic modulation was analyzed using Western blotting and CHIP-seq. Conditioned media was collected to culture CFSE-labeled T cells to assess proliferation. The nanoparticles were administered to mouse heart failure models induced by isoproterenol or angiotensin II and phenylephrine. Immune cell composition in mouse spleens and hearts was investigated by flow cytometry. Cardiac function was assessed by echocardiography and heart fibrosis was examined via immunohistochemistry and Western blotting.
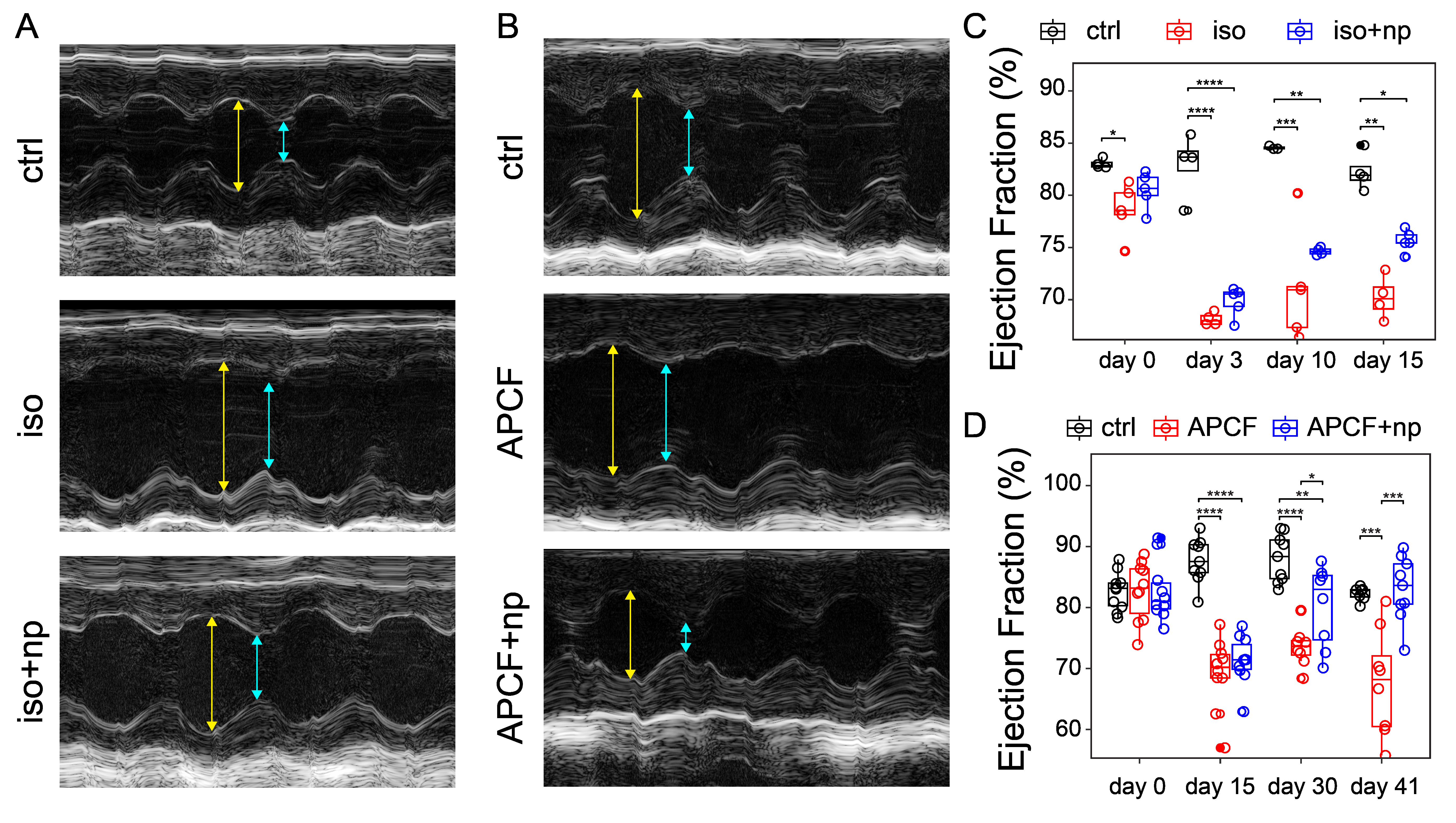
Results: Primary murine macrophages treated with our nanoparticles exhibited a dose-dependent T cell suppression in vitro. This effect was abrogated when an arginase inhibitor was administered together with the nanoparticles. The nanoparticles upregulated arginase I protein expression and arginase activity by increasing histone lactylation. In vivo testing with mouse heart injury models showed that the nanoparticles restored levels of effector and regulatory T cells in injured mice to non-injured levels. Additionally, the nanoparticles reduced cardiac fibrosis and restored ejection fraction. Furthermore, Western blotting of heart tissue lysate revealed that the nanoparticles downregulated the TGFb and p-SMAD signaling pathway.
Conclusion: Collectively, these findings highlight a novel therapeutic strategy for heart failure, addressing the gap of fibrosis treatment by targeting upstream immunological pathways.
Hypothesis: Cargo-less nanoparticles attenuate CD4+ T cell activation, expansion, and heart infiltration in experimental models of cardiomyopathy by regulating cross talk between macrophages and CD4+ T cells via L-arginine depletion in the spleen.
Methods: Spleen-targeting, cargo-less nanoparticles were synthesized from poly(L-lactide-co-glycolide) (PLLGA) and administered to murine and human macrophages in vitro. Arginase I expression and activity were assessed via Western blotting and arginase activity assay. Epigenetic modulation was analyzed using Western blotting and CHIP-seq. Conditioned media was collected to culture CFSE-labeled T cells to assess proliferation. The nanoparticles were administered to mouse heart failure models induced by isoproterenol or angiotensin II and phenylephrine. Immune cell composition in mouse spleens and hearts was investigated by flow cytometry. Cardiac function was assessed by echocardiography and heart fibrosis was examined via immunohistochemistry and Western blotting.
Results: Primary murine macrophages treated with our nanoparticles exhibited a dose-dependent T cell suppression in vitro. This effect was abrogated when an arginase inhibitor was administered together with the nanoparticles. The nanoparticles upregulated arginase I protein expression and arginase activity by increasing histone lactylation. In vivo testing with mouse heart injury models showed that the nanoparticles restored levels of effector and regulatory T cells in injured mice to non-injured levels. Additionally, the nanoparticles reduced cardiac fibrosis and restored ejection fraction. Furthermore, Western blotting of heart tissue lysate revealed that the nanoparticles downregulated the TGFb and p-SMAD signaling pathway.
Conclusion: Collectively, these findings highlight a novel therapeutic strategy for heart failure, addressing the gap of fibrosis treatment by targeting upstream immunological pathways.
More abstracts on this topic:
β1-adrenergic autoantibodies (β1-AA) augment macropinocytosis in CD4+ T cells, leading to the expansion of CD4+CD28− T cell subsets in heart failure.
Sun Fei, Yao Junyan, Li Bingjie, Zhang Suli, Liu Huirong
Adipocyte Enhancer Binding Protein 1 (AEBP1) Inhibition as a Potential Anti-Fibrotic Therapy in Heart FailureShankar Thirupura S, Calder Dallen, Sachse Frank, Kyriakopoulos Christos, Maneta Eleni, Srinivasan Harini, Tseliou Eleni, Navankasattusas Sutip, Selzman Craig, Boudina Sihem, Seidel Thomas, Visker Joseph, Lavine Kory, Drakos Stavros, Amrute Junedh, Polishchuk Georgiy, Lunde J Ty, Ling Jing, Ferrin Peter, Hamouche Rana, Feigle Dominik