Final ID: MDP1331
Extracellular Transcriptomics-driven Identification and Characterization of lncRNA LINC00989 in Heart Failure Progression
Abstract Body (Do not enter title and authors here): Introduction:
Heart failure (HF) poses a significant clinical challenge, necessitating a deeper understanding of its molecular mechanisms for effective therapeutic interventions. Long non-coding RNAs (lncRNAs) have emerged as key regulators in cardiovascular diseases. However, their role in HF progression, particularly in human pericytes and association with cardiac remodeling, remains poorly understood.
Hypothesis:
We hypothesize that LINC00989, a novel lncRNA identified through plasma extracellular vesicle (EV) transcriptomics of ADHF patients, plays a significant role in human pericytes towards its profibrotic function and contributes to HF remodeling.
Methods:
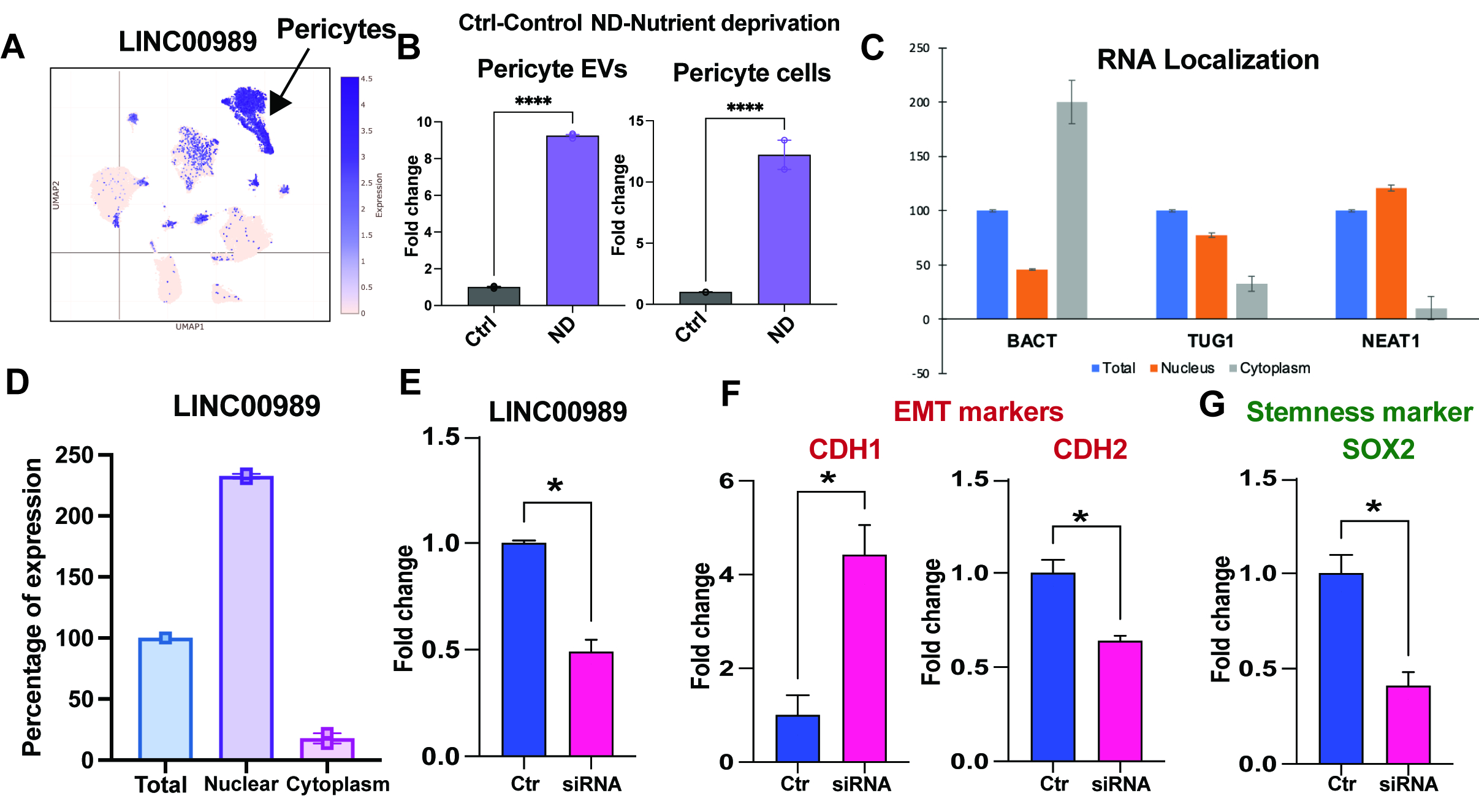
We characterized LINC00989 expression in primary human pericytes and its response to stressors mimicking HF. Cellular and extracellular fractionation experiments were performed to examine LINC00989 localization and its association with EVs. Loss-of-function experiments were conducted using RNA anti-sense oligos, while gain-of-function experiments involved overexpression vectors. Further downstream interactors were characterized using RNA sequencing analysis. Profibrotic effects of LINC00989 were investigated using adoptive transfer model with induced pluripotent stem cell-derived cardiac fibroblasts.
Results:
Our findings demonstrate high expression of LINC00989 in primary human pericytes, with differential regulation under stress conditions. Loss-of-function experiments revealed alterations in epithelial-mesenchymal transition and angiogenesis markers, implicating LINC00989 in maintaining pericyte phenotype. Gain-of-function experiments supported its role in modulating pericyte morphology. Additionally, LINC00989 indicated a profibrotic role on cardiac fibroblasts.
Conclusion:
These findings underscore the significance of LINC00989 in human pericyte function and its potential as a therapeutic target in HF progression. Understanding its molecular mechanisms could lead to novel therapeutic interventions in heart failure. Further studies are needed to validate these findings and explore clinical implications.
Heart failure (HF) poses a significant clinical challenge, necessitating a deeper understanding of its molecular mechanisms for effective therapeutic interventions. Long non-coding RNAs (lncRNAs) have emerged as key regulators in cardiovascular diseases. However, their role in HF progression, particularly in human pericytes and association with cardiac remodeling, remains poorly understood.
Hypothesis:
We hypothesize that LINC00989, a novel lncRNA identified through plasma extracellular vesicle (EV) transcriptomics of ADHF patients, plays a significant role in human pericytes towards its profibrotic function and contributes to HF remodeling.
Methods:
We characterized LINC00989 expression in primary human pericytes and its response to stressors mimicking HF. Cellular and extracellular fractionation experiments were performed to examine LINC00989 localization and its association with EVs. Loss-of-function experiments were conducted using RNA anti-sense oligos, while gain-of-function experiments involved overexpression vectors. Further downstream interactors were characterized using RNA sequencing analysis. Profibrotic effects of LINC00989 were investigated using adoptive transfer model with induced pluripotent stem cell-derived cardiac fibroblasts.
Results:
Our findings demonstrate high expression of LINC00989 in primary human pericytes, with differential regulation under stress conditions. Loss-of-function experiments revealed alterations in epithelial-mesenchymal transition and angiogenesis markers, implicating LINC00989 in maintaining pericyte phenotype. Gain-of-function experiments supported its role in modulating pericyte morphology. Additionally, LINC00989 indicated a profibrotic role on cardiac fibroblasts.
Conclusion:
These findings underscore the significance of LINC00989 in human pericyte function and its potential as a therapeutic target in HF progression. Understanding its molecular mechanisms could lead to novel therapeutic interventions in heart failure. Further studies are needed to validate these findings and explore clinical implications.
More abstracts on this topic:
Ca2+/Calmodulin Dependent & Independent Regulation of Obscurin Kinase 1
Gonzales Rex, Takagi Yasuharu, Wright Nathan, Kontrogianni-konstantopoulos Aikaterini
Activation of TRPA1 with allyl isothiocyanate prevents age-related cardiac diastolic dysfunctionQian Chunqi, Fernandez Zachary, Wang Donna, Ma Shuangtao