Final ID: MP2675
Increased Carnitine Palmitoyltransferase 1a Expression is a Critical Cardioprotective Response to Pathological Stress That Suppresses Gene Programs For Adverse Remodeling Through Non-Conical Signaling and Enables Rescue by Gene Delivery
Abstract Body (Do not enter title and authors here): Background: Carnitine palmitoyl transferase 1 (CPT1) is a rate-limiting enzyme for long chain fatty acid oxidation (FAO). In adult hearts, CPT1b predominates, while CPT1a is co-expressed at lower levels. Pathological stress on the heart induces CPT1a expression, coinciding with a reduction in FAO.
Research Questions/Hypothesis: Is the upregulation of CPT1a maladaptive or an adaptive response to chronic pressure overload and how do changes in CPT1a expression alter intracellular signaling and cardiac metabolism?
Methods: Mice were subjected to afterload stress via transverse aortic constriction (TAC) or sham surgery (sham) with cardiac-specific CPT1a knockdown (csCPT1a ko) or cardiac-specific, AAV9-mediated CPT1a overexpression (AAV9.cTNTN.Cpt1a).
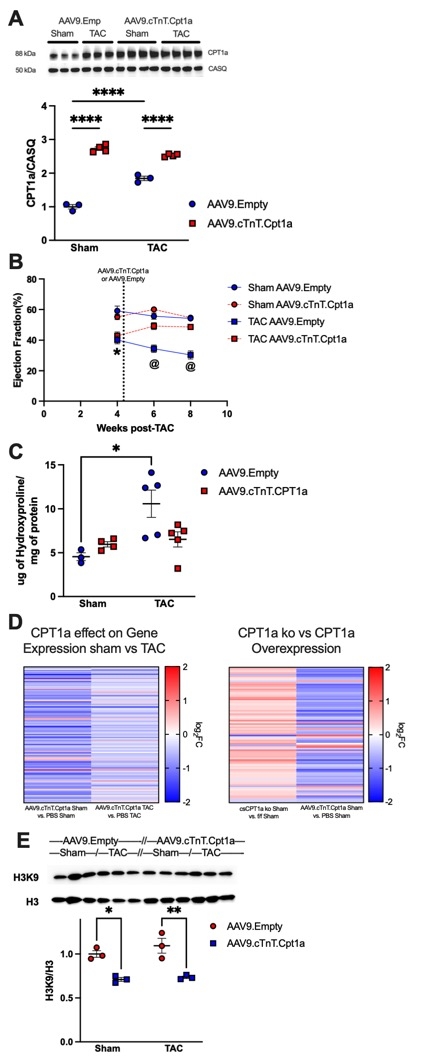
Results: Preventing CPT1a upregulation in response to TAC in cardiac specific CPT1a knockout mice (csCPT1a ko) exacerbated adverse remodeling, induced severe dysfunction, and increased mortality. In contrast, CPT1a overexpression (2.8 fold, Panel A) prior to TAC, attenuated impaired ejection fraction (EF, by 54%) vs. control TAC hearts (n=5-8, p<0.05). Delivery of AAV9.cTnT.Cpt1a 4 wks after TAC surgery led to significant rescue of EF (Panel B) and reversed hydroxyproline accumulation (Panel C), while also mitigating the exacerbated dysfunction of csCPT1a ko TAC hearts. RNA-seq revealed a novel function of CPT1a in suppressing gene expression programs for hypertrophic, profibrotic and cell death pathways in both sham and TAC hearts (Panel D) while loss of CPT1a induced expression (n=3-4). The changes in gene expression patterns occurred irrespective of changes in FAO but were proportional to CPT1a changes. Histone H3K9 acetylation was significantly reduced in hearts from both sham and TAC mice treated with AAV9.cTnT.Cpt1a (Panel E) while sirtuin 1 expression was increased.
Conclusions: CPT1a upregulation in failing myocardium is a critical cardioprotective adaptation to pathological stress. The effects of CPT1a expression in the heart extend beyond FAO to include non-canonical regulation of cardiac gene programs, even in the absence of pathological stress. Evidence from cardiac specific, AAV-mediated CPT1a overexpression supports potential for gene therapy to limit progression of heart failure.
Research Questions/Hypothesis: Is the upregulation of CPT1a maladaptive or an adaptive response to chronic pressure overload and how do changes in CPT1a expression alter intracellular signaling and cardiac metabolism?
Methods: Mice were subjected to afterload stress via transverse aortic constriction (TAC) or sham surgery (sham) with cardiac-specific CPT1a knockdown (csCPT1a ko) or cardiac-specific, AAV9-mediated CPT1a overexpression (AAV9.cTNTN.Cpt1a).
Results: Preventing CPT1a upregulation in response to TAC in cardiac specific CPT1a knockout mice (csCPT1a ko) exacerbated adverse remodeling, induced severe dysfunction, and increased mortality. In contrast, CPT1a overexpression (2.8 fold, Panel A) prior to TAC, attenuated impaired ejection fraction (EF, by 54%) vs. control TAC hearts (n=5-8, p<0.05). Delivery of AAV9.cTnT.Cpt1a 4 wks after TAC surgery led to significant rescue of EF (Panel B) and reversed hydroxyproline accumulation (Panel C), while also mitigating the exacerbated dysfunction of csCPT1a ko TAC hearts. RNA-seq revealed a novel function of CPT1a in suppressing gene expression programs for hypertrophic, profibrotic and cell death pathways in both sham and TAC hearts (Panel D) while loss of CPT1a induced expression (n=3-4). The changes in gene expression patterns occurred irrespective of changes in FAO but were proportional to CPT1a changes. Histone H3K9 acetylation was significantly reduced in hearts from both sham and TAC mice treated with AAV9.cTnT.Cpt1a (Panel E) while sirtuin 1 expression was increased.
Conclusions: CPT1a upregulation in failing myocardium is a critical cardioprotective adaptation to pathological stress. The effects of CPT1a expression in the heart extend beyond FAO to include non-canonical regulation of cardiac gene programs, even in the absence of pathological stress. Evidence from cardiac specific, AAV-mediated CPT1a overexpression supports potential for gene therapy to limit progression of heart failure.
More abstracts on this topic:
Cardiac Branched-Chain Amino Acid Metabolic Dysfunction in a Novel Model of Diabetic Cardiomyopathy
Nagao Manabu, Asakura Junko, Hosooka Tetsuya, Kuwahara Naoya, Kaneshiro Kenta, Tanaka Hidekazu, Ishida Tatsuro, Otake Hiromasa, Shinohara Masakazu
Ablation of Nephron Tubule Cell-Specific Npr1 Triggers High Blood Pressure and DysfunctionNeelamegam Kandasamy, Ramasamy Chandramohan, Pandey Kailash