Final ID: MP2782
Real-World Outcomes of Mavacamten Monotherapy in Obstructive Hypertrophic Cardiomyopathy: Symptomatic and Hemodynamic Improvements Without Increased Adverse Events
Abstract Body (Do not enter title and authors here): Background: MARVEL-HCM is a US multicenter study assessing the safety and effectiveness of mavacamten in real-world academic and community practices. Mavacamten is a first-in-class cardiac myosin inhibitor (CMI) and the only CMI currently approved in over 50 countries for use in patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM). Modest evidence exists describing the effectiveness and safety of mavacamten monotherapy.
Aims: To describe the real-world outcomes of patients using mavacamten as monotherapy for oHCM.
Methods: Patient-level data from medical records at 5 sites were analyzed to describe mavacamten monotherapy, defined as monotherapy at baseline or after background HCM therapy (beta-blockers [BB], calcium channel blockers [CCB], disopyramide) discontinuation within 12 weeks(wks) of mavacamten initiation and without reinitiation, up to 60 wks. A minimum of 12 wks follow-up was required for inclusion. Patient characteristics, echocardiographic data, and safety are reported at baseline and follow-up visits.
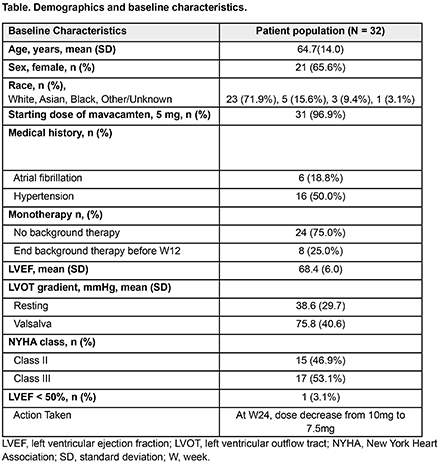
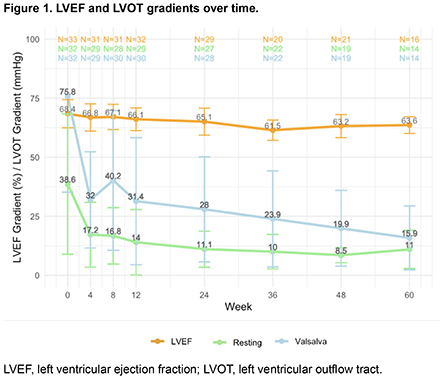
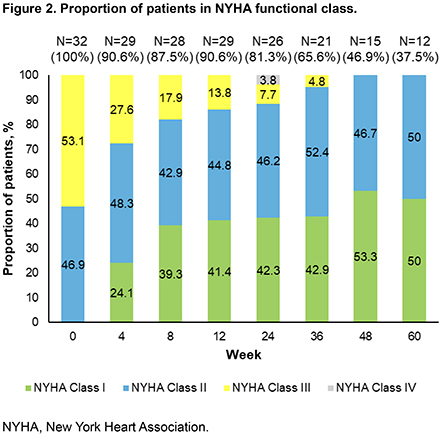
Results: The monotherapy cohort comprised 13.4% (32/239) of patients: 24 were on mavacamten monotherapy at initiation, and 8 discontinued background therapy. Baseline characteristics included: NYHA Class II (46.9%) and III (53.1%), female (66%), hypertension (50%), and atrial fibrillation (AF) (18%) (Table 1). Median follow-up and duration of mavacamten monotherapy were 48 wks (range 12-60 wks) and 57 wks (range 14-60 wks), respectively. Resting left ventricular outflow tract gradient (LVOTg) was ≤ 30 mmHg in 93%, 91% and 100% of patients at Wk 24, 36, and 60. Valsalva LVOTg was ≤ 30 mmHg in 61%, 77% and 86% of patients at Wk 24, 36, and 60 (Fig 1). No patients were in NYHA Class III at wk 60, and ~50% of patients were NYHA Class I by wk 48 (Fig 2). Mean reduction in LVEF was 4.8% over 60 wks. One patient had LVEF < 50% at Wk 20, resulting in a dose reduction (10 to 7.5 mg). Safety events included 3 hospitalizations due to AF ablation (n=1), viral gastrointestinal illness (n=1) and heart failure (n=1). Demographic, clinical characteristics, and outcomes were similar across the mavacamten monotherapy cohort, mavacamten in combination with background HCM therapy (n=207), and the overall cohort of MARVEL-HCM (N=239).
Conclusions: Mavacamten monotherapy was effective, safe, and consistent with the overall MARVEL cohort, as well as previously reported monotherapy cohorts from pivotal, long-term, and real-world evidence.
Aims: To describe the real-world outcomes of patients using mavacamten as monotherapy for oHCM.
Methods: Patient-level data from medical records at 5 sites were analyzed to describe mavacamten monotherapy, defined as monotherapy at baseline or after background HCM therapy (beta-blockers [BB], calcium channel blockers [CCB], disopyramide) discontinuation within 12 weeks(wks) of mavacamten initiation and without reinitiation, up to 60 wks. A minimum of 12 wks follow-up was required for inclusion. Patient characteristics, echocardiographic data, and safety are reported at baseline and follow-up visits.
Results: The monotherapy cohort comprised 13.4% (32/239) of patients: 24 were on mavacamten monotherapy at initiation, and 8 discontinued background therapy. Baseline characteristics included: NYHA Class II (46.9%) and III (53.1%), female (66%), hypertension (50%), and atrial fibrillation (AF) (18%) (Table 1). Median follow-up and duration of mavacamten monotherapy were 48 wks (range 12-60 wks) and 57 wks (range 14-60 wks), respectively. Resting left ventricular outflow tract gradient (LVOTg) was ≤ 30 mmHg in 93%, 91% and 100% of patients at Wk 24, 36, and 60. Valsalva LVOTg was ≤ 30 mmHg in 61%, 77% and 86% of patients at Wk 24, 36, and 60 (Fig 1). No patients were in NYHA Class III at wk 60, and ~50% of patients were NYHA Class I by wk 48 (Fig 2). Mean reduction in LVEF was 4.8% over 60 wks. One patient had LVEF < 50% at Wk 20, resulting in a dose reduction (10 to 7.5 mg). Safety events included 3 hospitalizations due to AF ablation (n=1), viral gastrointestinal illness (n=1) and heart failure (n=1). Demographic, clinical characteristics, and outcomes were similar across the mavacamten monotherapy cohort, mavacamten in combination with background HCM therapy (n=207), and the overall cohort of MARVEL-HCM (N=239).
Conclusions: Mavacamten monotherapy was effective, safe, and consistent with the overall MARVEL cohort, as well as previously reported monotherapy cohorts from pivotal, long-term, and real-world evidence.
More abstracts on this topic:
Aficamten is safe and effective in oHCM with comorbidities obesity, hypertension, and diabetes: a SEQUOIA-HCM sub-study
Lee Matthew, Malik Fady, Kupfer Stuart, Wohltman Amy, Coats Caroline, Abraham Theodore, Claggett Brian, Maron Martin, Miao Zi, Meder Benjamin, Olivotto Iacopo, Heitner Stephen, Jacoby Daniel
A Hypertrophic Cardiomyopathy Polygenic Score Modifies Penetrance of Pathogenic Hypertrophic and Dilated Cardiomyopathy Variants in Opposite DirectionsAbramowitz Sarah, Hoffman-andrews Lily, Depaolo John, Judy Renae, Owens Anjali, Damrauer Scott, Levin Michael