Final ID: MP1042
Aficamten is safe and effective in oHCM with comorbidities obesity, hypertension, and diabetes: a SEQUOIA-HCM sub-study
Research Questions/Hypothesis: Efficacy of aficamten in patients with oHCM and comorbidities.
Methods/Approach: SEQUOIA-HCM (NCT05186818) randomized 282 adults with symptomatic oHCM to aficamten or placebo for 24 weeks. Participants were grouped by obesity (body mass index [BMI] ≥30 kg/m2), hypertension (history or average screening/baseline systolic blood pressure ≥140 or diastolic blood pressure ≥90 mmHg), and diabetes (type 1, type 2, or unspecified per history). Baseline characteristics and treatment effects on peak oxygen uptake (pVO2) and secondary endpoints were compared across comorbidity groups.
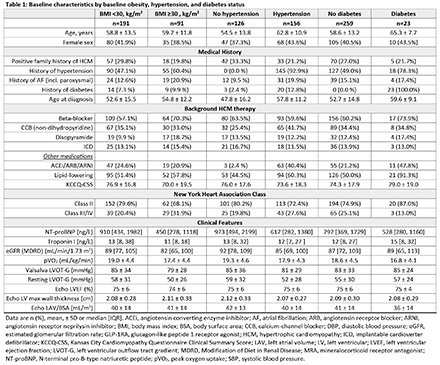
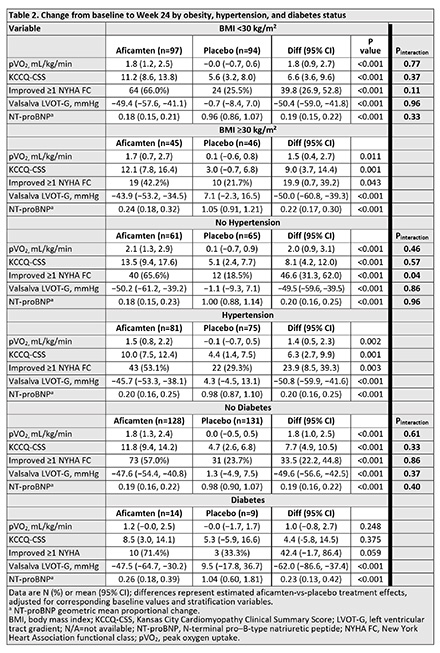
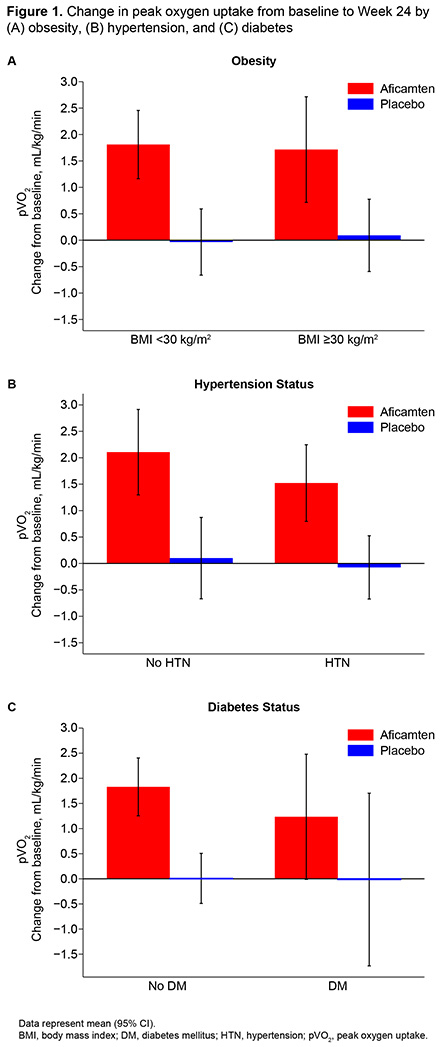
Results/Data: At baseline, 32% had obesity, 55% had hypertension, and 8% had diabetes; 24% had 2 comorbidities, and 3% had all 3 comorbidities. At baseline, obesity was associated with lower Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS), N-terminal pro-B-type natriuretic peptide (NT-proBNP), pVO2, and resting left ventricular outflow tract gradient (LVOT-G), and higher use of beta-blockers and disopyramide (Table 1). At baseline, hypertension was associated with lower NT-proBNP, pVO2, and resting LVOT-G; older age; more atrial fibrillation and diabetes; and more use of non-dihydropyridine calcium-channel blockers and renin angiotensin system blockers. At baseline, diabetes was associated with older age, hypertension, higher KCCQ-CSS, lower NT-proBNP, and pVO2, and more use of renin angiotensin system blockers. Despite baseline differences, aficamten treatment compared to placebo consistently improved pVO2, KCCQ-CSS, Valsalva LVOT-G, and NT-proBNP independent of comorbid status (all interaction p-values >0.05) (Figure 1; Table 2). The incidence of serious adverse events and left ventricular ejection fraction <50% were similar across subgroups, including when grouped by treatment arm.
Conclusion(s): Comorbidities, particularly obesity and hypertension, are common in patients with oHCM. Aficamten treatment showed consistent clinical efficacy regardless of comorbidity status, supporting its use across a broad patient population.
- Lee, Matthew ( University of Glasgow , Glasgow , United Kingdom )
- Malik, Fady ( Cytokinetics Inc. , South San Francisco , California , United States )
- Kupfer, Stuart ( Cytokinetics Inc. , South San Francisco , California , United States )
- Wohltman, Amy ( Cytokinetics Inc. , South San Francisco , California , United States )
- Coats, Caroline ( University og Glasgow , Glasgow , United Kingdom )
- Abraham, Theodore ( Univ of California at San Francisco , San Francisco , California , United States )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Maron, Martin ( Lahey Hospital and Medical Center , Burlington , Massachusetts , United States )
- Miao, Zi ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Meder, Benjamin ( University of Heidelberg , Heidelberg , Germany )
- Olivotto, Iacopo ( University of Florence , Florence , Italy )
- Heitner, Stephen ( Cytokinetics Inc. , South San Francisco , California , United States )
- Jacoby, Daniel ( Cytokinetics Inc. , South San Francisco , California , United States )
Meeting Info:
Session Info:
Targeting the Thickened Heart: Advances in Hypertrophic Cardiomyopathy Therapy
Saturday, 11/08/2025 , 10:45AM - 12:00PM
Moderated Digital Poster Session
More abstracts on this topic:
Prendergast Heather, Khosla Shaveta, Kitsiou Spyros, Petzel Gimbar Renee, Freels Sally, Sanders Anissa, Daviglus Martha, Carter Barry, Del Rios Marina, Heinert Sara
A cerebrovascular longitudinal atlas: different rates of morphological change in aneurysm patients associated with hypertension and diabetesChien Aichi, Salamon Noriko, Vinuela Fernando, Szeder Viktor, Colby Geoffrey, Jahan Reza, Boyle Noel, Villablanca Juan, Duckwiler Gary
More abstracts from these authors:
Wang Xiaowen, Nassif Michael, Olivotto Iacopo, Jacoby Daniel, Heitner Stephen, Wohltman Amy, Solomon Scott, Hegde Sheila, Pabon Maria, Abraham Theodore, Barriales-villa Roberto, Claggett Brian, Coats Caroline, Maron Martin, Masri Ahmad, Meder Benjamin
Improvement in Echocardiographic Measures of Diastolic Function Reflects Improved Exercise Performance in Obstructive Hypertrophic Cardiomyopathy: Insights From the SEQUOIA-HCM TrialLu Henri, Olivotto Iacopo, Jacoby Daniel, Heitner Stephen, Kupfer Stuart, Malik Fady, Wohltman Amy, Solomon Scott, Hegde Sheila, Bart Nicole, Claggett Brian, Abraham Theodore, Coats Caroline, Lee Matthew, Lewis Gregory, Maron Martin, Masri Ahmad